
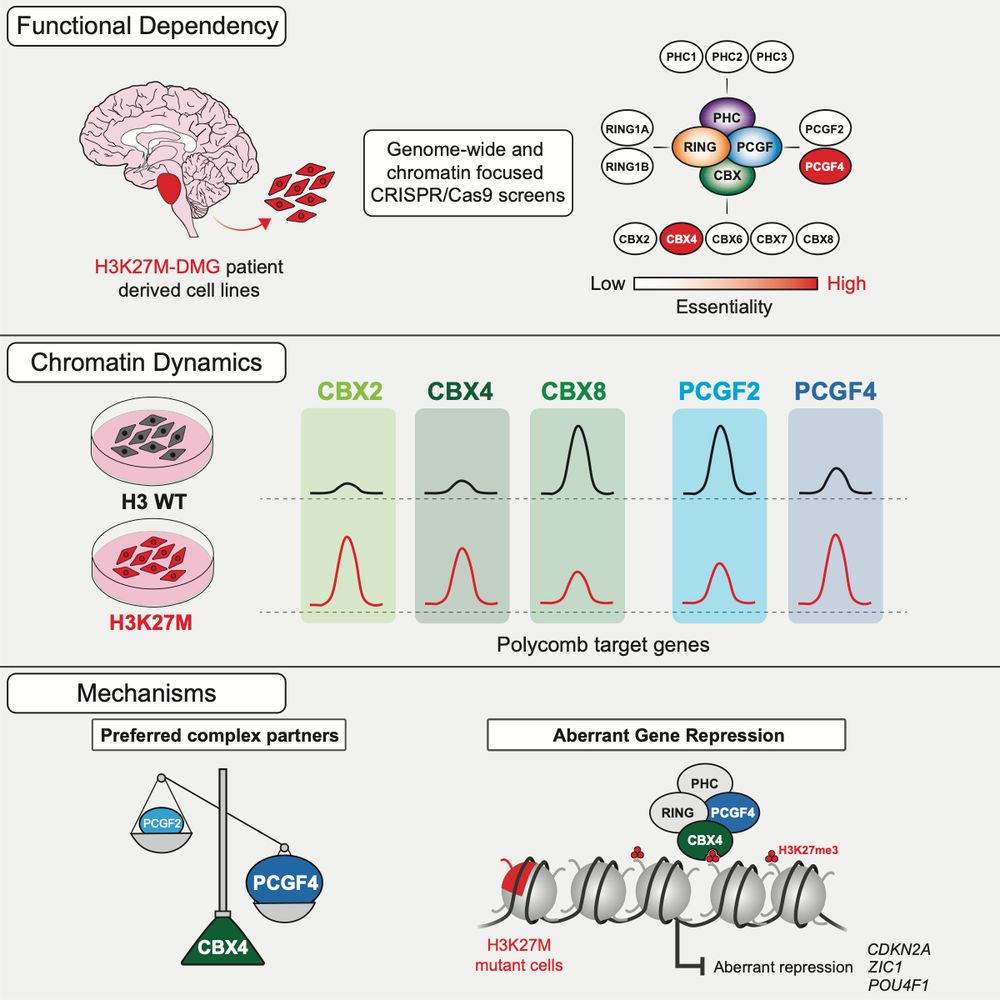
You were told that AEBP2 promotes PRC2 activity on chromatin.
We found the opposite: the most prevalent AEBP2 isoform inhibits PRC2 activity.
👉 surl.li/cgwqcq
A thread 🧵
Registration link: us06web.zoom.us/webinar/regi...
Registration link: us06web.zoom.us/webinar/regi...
Please share this with your followers to let them know you will speaking, and to register & submit today to join us
🔗 bit.ly/4rC6B45

Please share this with your followers to let them know you will speaking, and to register & submit today to join us
🔗 bit.ly/4rC6B45
For years, the assumptions on disease mechanisms were simple:
➡️ SS18-SSX works by hijacking SWI/SNF chromatin remodeling activity
Our new study shows that assumption was wrong 🧵👇
www.biorxiv.org/content/10.6...
For years, the assumptions on disease mechanisms were simple:
➡️ SS18-SSX works by hijacking SWI/SNF chromatin remodeling activity
Our new study shows that assumption was wrong 🧵👇
www.biorxiv.org/content/10.6...
First up, on Jan 30th we will be joined by @lucas.farnunglab.com from @harvardmed.bsky.social 🔬
Shortly after, on Feb 13th we will be hosting @heardlab.bsky.social from @crick.ac.uk 🧬
Registration details 👇


First up, on Jan 30th we will be joined by @lucas.farnunglab.com from @harvardmed.bsky.social 🔬
Shortly after, on Feb 13th we will be hosting @heardlab.bsky.social from @crick.ac.uk 🧬
Registration details 👇
@adrianbracken.bsky.social @davidovichlab.bsky.social and colleagues
www.embopress.org/doi/full/10....

@adrianbracken.bsky.social @davidovichlab.bsky.social and colleagues
www.embopress.org/doi/full/10....
@ucddublin.bsky.social @tcddublin.bsky.social @ucd-sbbs.bsky.social
New Outlook, Review and Research articles online now at Genes & Development.
Click on the link to learn more:
➡️ https://genesdev.cshlp.org/content/39/21-22.toc

@ucddublin.bsky.social @tcddublin.bsky.social @ucd-sbbs.bsky.social
You were told that AEBP2 promotes PRC2 activity on chromatin.
We found the opposite: the most prevalent AEBP2 isoform inhibits PRC2 activity.
👉 surl.li/cgwqcq
A thread 🧵
You were told that AEBP2 promotes PRC2 activity on chromatin.
We found the opposite: the most prevalent AEBP2 isoform inhibits PRC2 activity.
👉 surl.li/cgwqcq
A thread 🧵
#CancerResearch #Epigenetics #Chromatin #Lymphoma

#CancerResearch #Epigenetics #Chromatin #Lymphoma

#CancerResearch #Epigenetics #Chromatin #Lymphoma
This exciting work on chromatinopathies 🧬 was in collab with @adrianbracken.bsky.social and spearheaded by Orla Deevy.
@ucddublin.bsky.social @ucd-sbbs.bsky.social
www.ucd.ie/newsandopini...

This exciting work on chromatinopathies 🧬 was in collab with @adrianbracken.bsky.social and spearheaded by Orla Deevy.
@ucddublin.bsky.social @ucd-sbbs.bsky.social
www.ucd.ie/newsandopini...
Join us at @tcddublin.bsky.social on May 28th for this event featuring keynote, new PI and PhD/postdoc talks and posters!
allirelandchromatinconsortium.ie/annual-meeti...

Join us at @tcddublin.bsky.social on May 28th for this event featuring keynote, new PI and PhD/postdoc talks and posters!
allirelandchromatinconsortium.ie/annual-meeti...

Join us at @tcddublin.bsky.social on May 28th for this event featuring keynote, new PI and PhD/postdoc talks and posters!
allirelandchromatinconsortium.ie/annual-meeti...
We show that JARID2 and PALI1 mimic H3K27me3 to antagonise PRC2 in vivo and restrict the spread of Polycomb domains.
🧵
www.biorxiv.org/content/10.1...
We show that JARID2 and PALI1 mimic H3K27me3 to antagonise PRC2 in vivo and restrict the spread of Polycomb domains.
🧵
www.biorxiv.org/content/10.1...
Register here: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social
Register here: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social
Keep an eye out for job ads from them soon 👀
chromosome.ie
Keep an eye out for job ads from them soon 👀
Registration details here: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social

Registration details here: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social
genetics.org.uk/events/epige...

genetics.org.uk/events/epige...
Don't miss this chance to share your research & network with experts in the field.
genetics.org.uk/events/epige...

Don't miss this chance to share your research & network with experts in the field.
genetics.org.uk/events/epige...
@davidlabmsk.bsky.social will join us for her webinar on 'Turning back the clock: Targeting a new metabolism-epigenetic axis in T-cells.'
Registration: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social

@davidlabmsk.bsky.social will join us for her webinar on 'Turning back the clock: Targeting a new metabolism-epigenetic axis in T-cells.'
Registration: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social
We will be joined by Luciano Di Croce for his webinar on 'Dissecting Dynamics of Chromatin Function in Cell Differentiation and Cancer'.
Register here: us06web.zoom.us/webinar/regi...
@activemotifusa.bsky.social



