

www.statnews.com/2025/04/05/c...
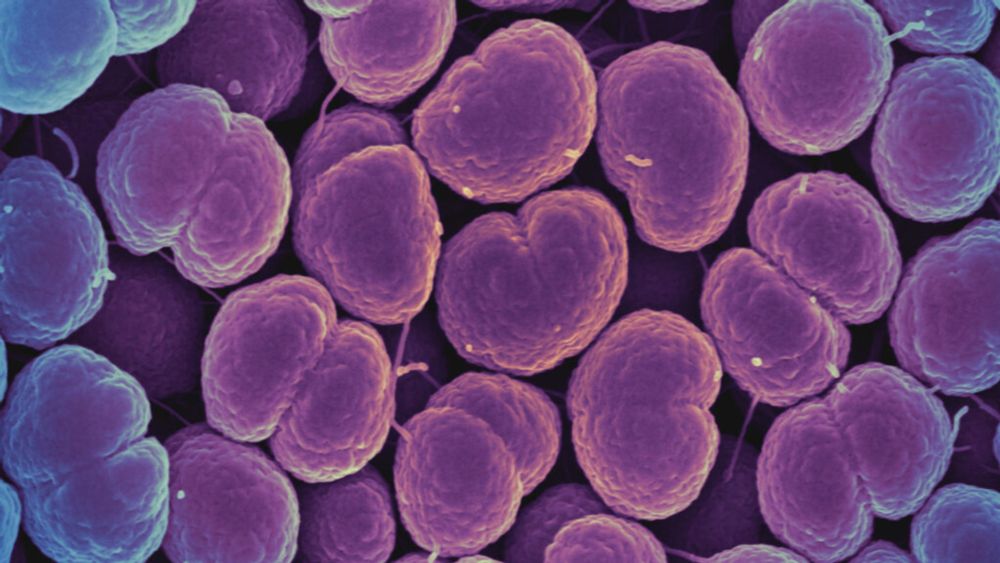
www.statnews.com/2025/04/05/c...

Got a PhD in disease evolution, or something similarly hardcore? Know your way around phylodynamics? Good. We need you.
If you’re up for real-world research getting hands-on with fieldwork, sequencing, and making sense of viral evolution, apply here: jobs.inrae.fr/en/ot-25456.
Got a PhD in disease evolution, or something similarly hardcore? Know your way around phylodynamics? Good. We need you.
If you’re up for real-world research getting hands-on with fieldwork, sequencing, and making sense of viral evolution, apply here: jobs.inrae.fr/en/ot-25456.

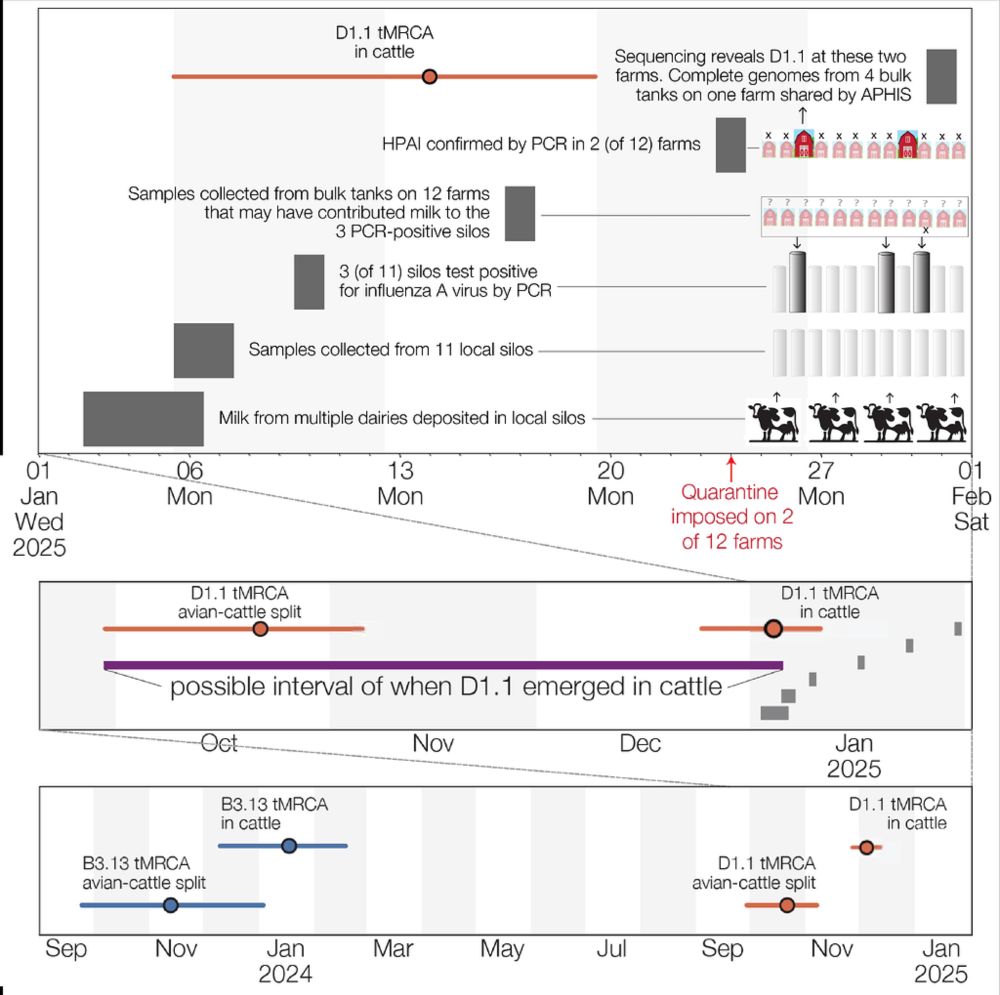
Our latest study on HPAI in France and risk mapping has just been published doi.org/10.1371/jour...
@epidesa.bsky.social @inrae-france.bsky.social @anses-fr.bsky.social ENVT ULB
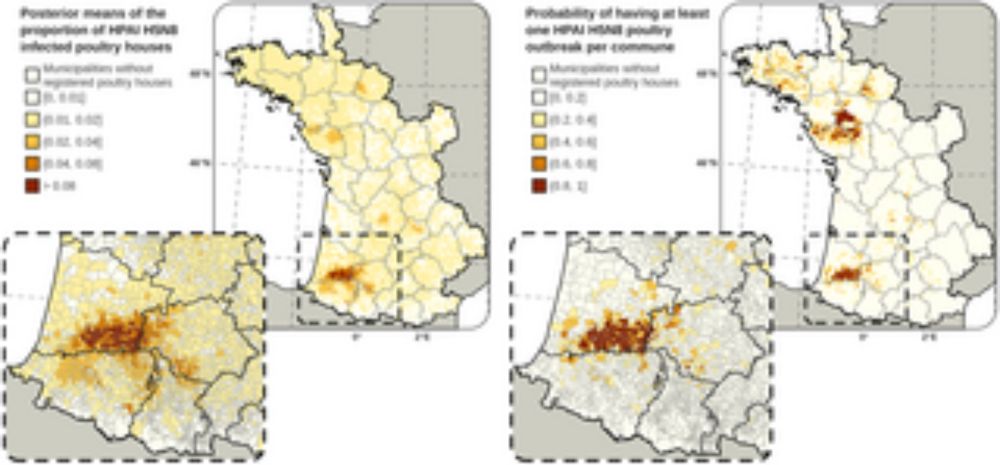
Our latest study on HPAI in France and risk mapping has just been published doi.org/10.1371/jour...
@epidesa.bsky.social @inrae-france.bsky.social @anses-fr.bsky.social ENVT ULB
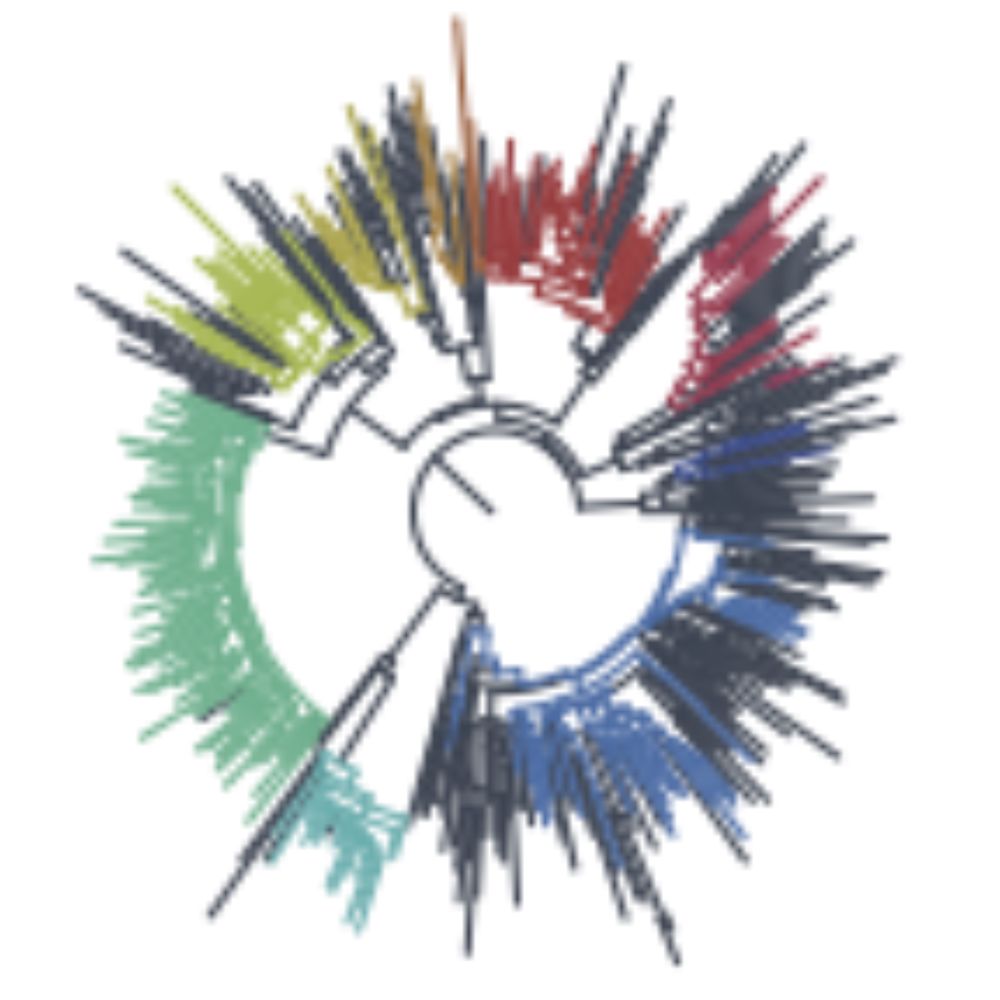
And the answer is yes.
In SRR29377776 from June 2024, collected from a mouse on, presumably, a farm.
Data:
www.ncbi.nlm.nih.gov/sra/?term=SR...
And the answer is yes.
In SRR29377776 from June 2024, collected from a mouse on, presumably, a farm.
Data:
www.ncbi.nlm.nih.gov/sra/?term=SR...

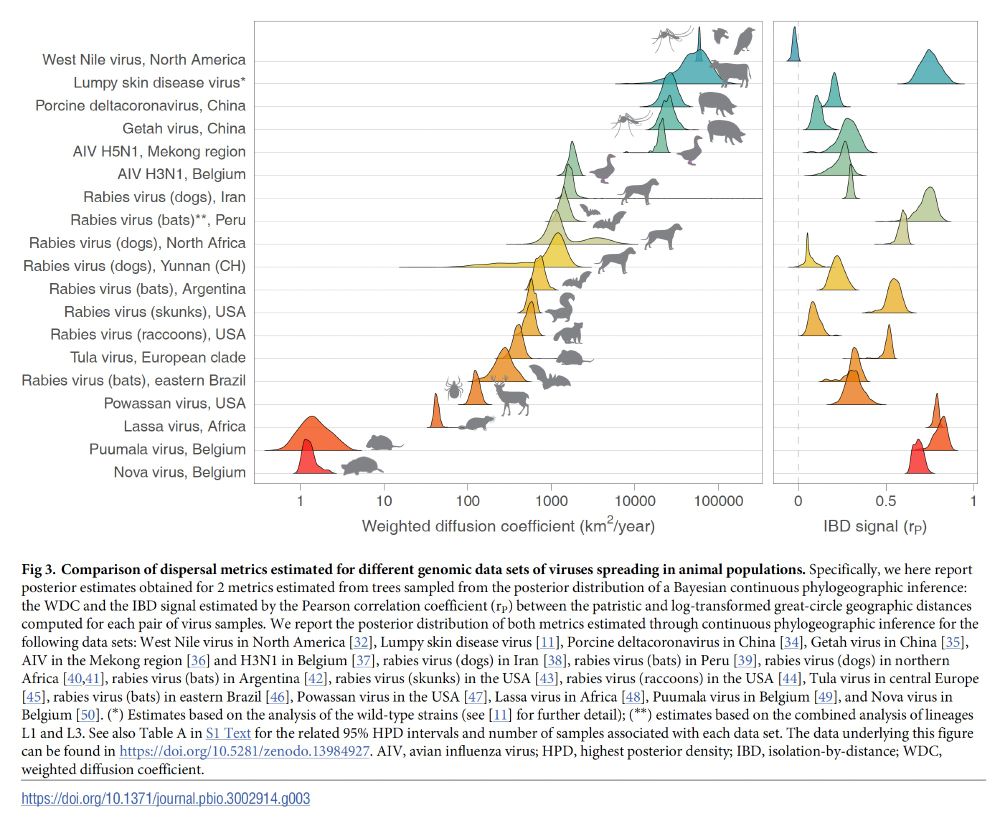

journals.plos.org/plosbiology/...

journals.plos.org/plosbiology/...


