Anamaria Elek
@aelek.bsky.social
190 followers
400 following
15 posts
Postdoc @ Kaessmann and Sasse labs @zmbh.uni-heidelberg.de
Previously PhD @ Sebé-Perdós lab @crg.eu
Interested in regulatory genomics, evolution, machine learning, and especially the combination of all of the above.
https://anamaria.elek.hr/
Posts
Media
Videos
Starter Packs
Reposted by Anamaria Elek
Reposted by Anamaria Elek
Kaessmann Lab
@kaessmannlab.bsky.social
· Jul 16
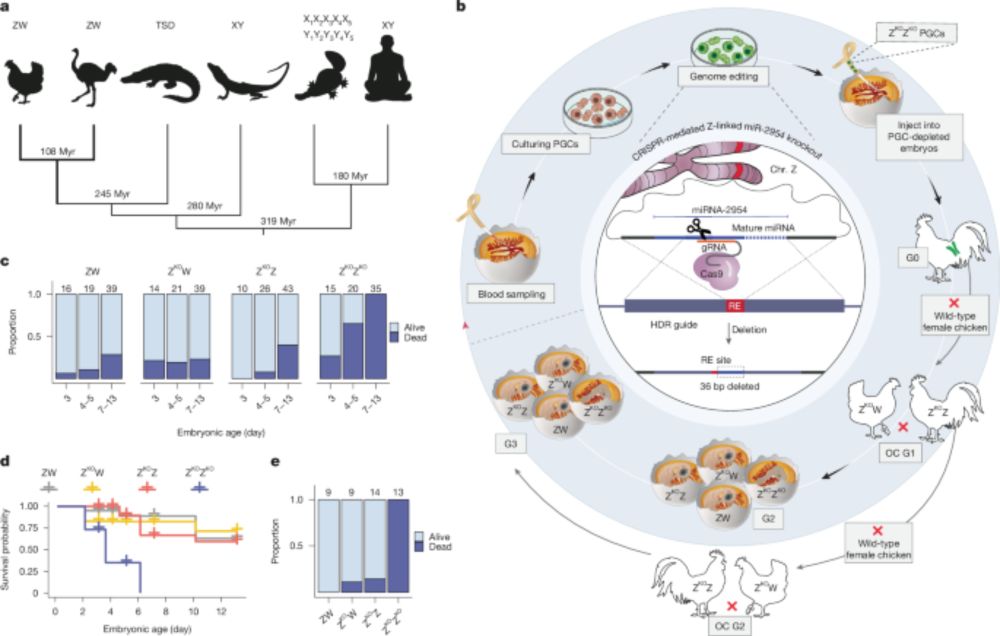
A male-essential miRNA is key for avian sex chromosome dosage compensation - Nature
Birds have evolved a unique sex chromosome dosage compensation mechanism involving the male-biased microRNA (miR-2954), which is essential for male survival by regulating the expression of dosage-sens...
www.nature.com
Anamaria Elek
@aelek.bsky.social
· Jul 6
Anamaria Elek
@aelek.bsky.social
· Jul 6
Anamaria Elek
@aelek.bsky.social
· Jul 6
Anamaria Elek
@aelek.bsky.social
· Jul 6
Anamaria Elek
@aelek.bsky.social
· Jul 6
Decoding cnidarian cell type gene regulation
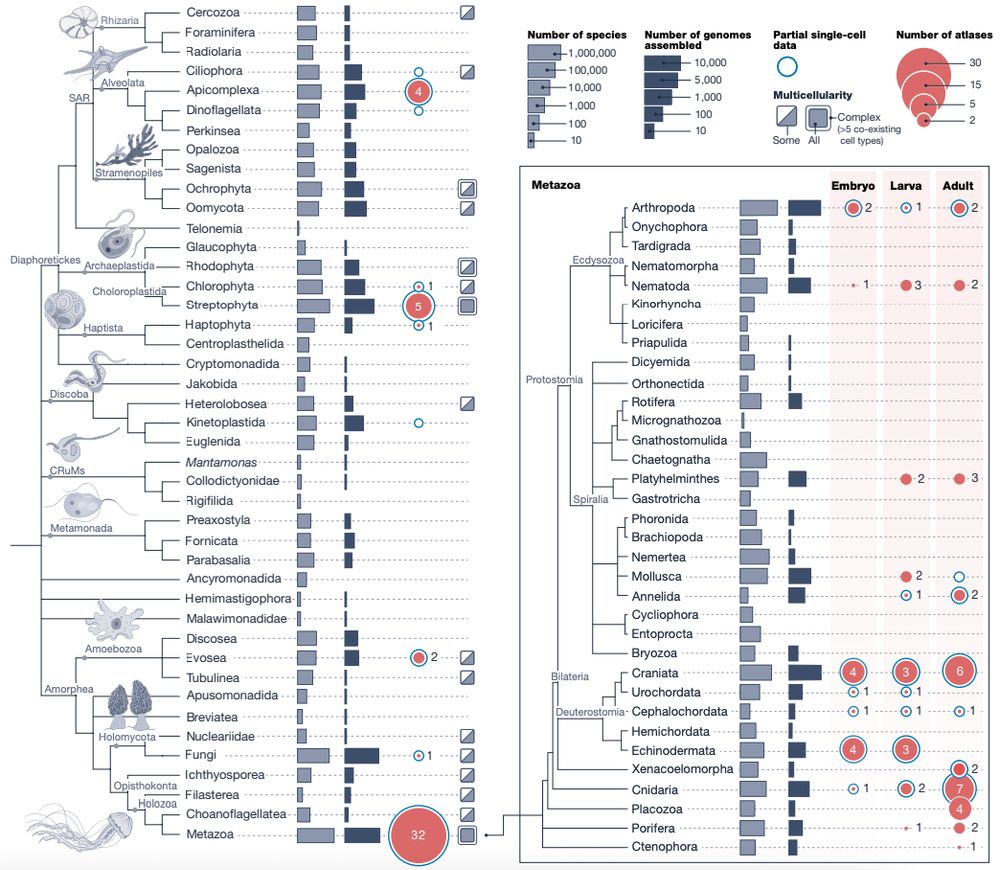
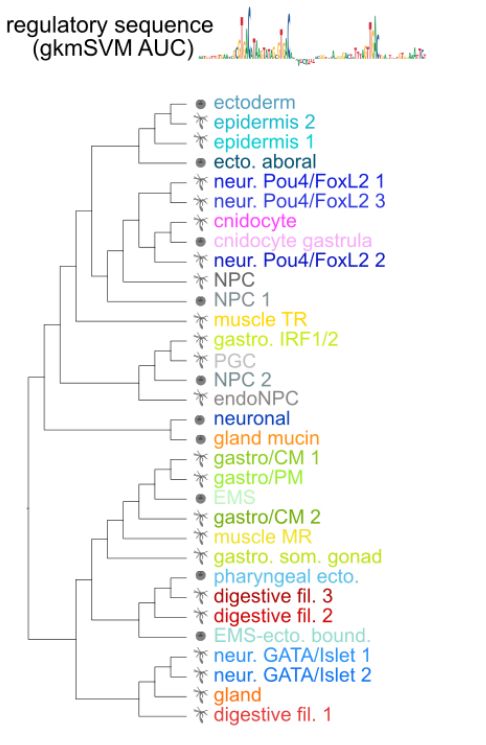
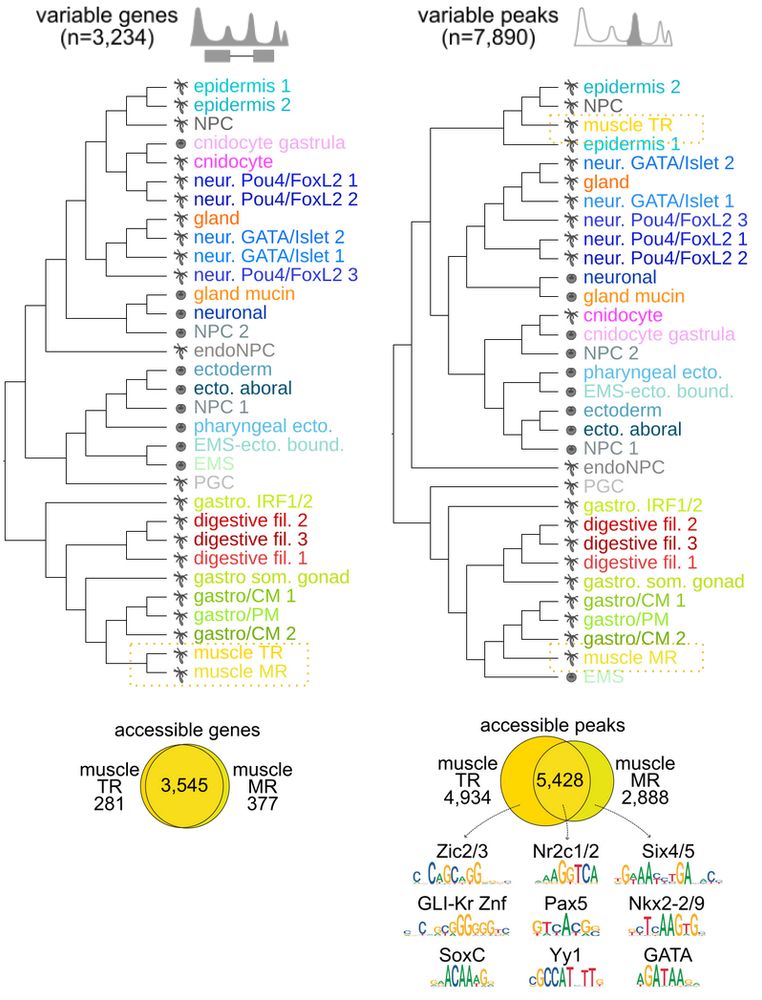
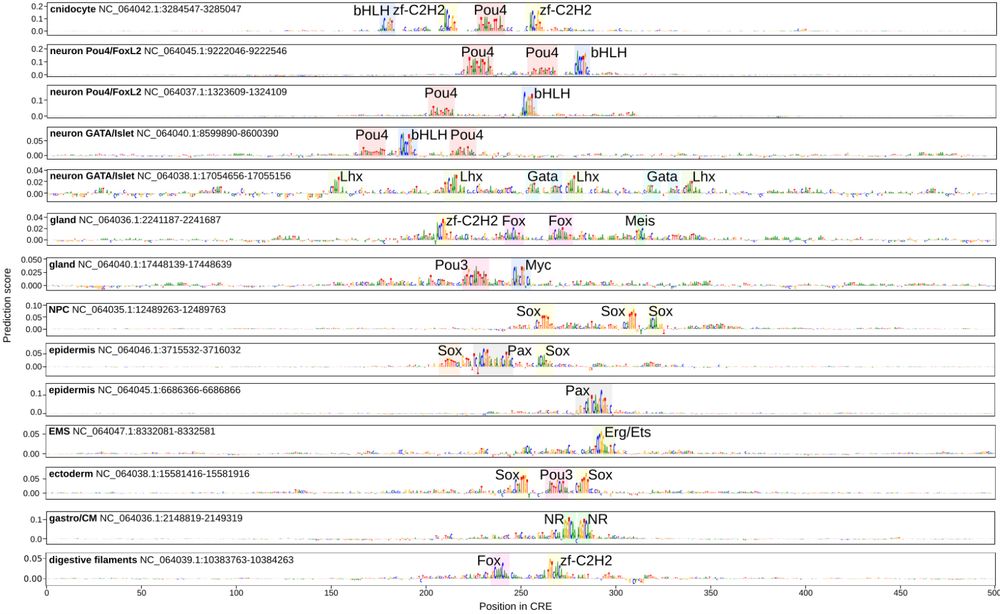
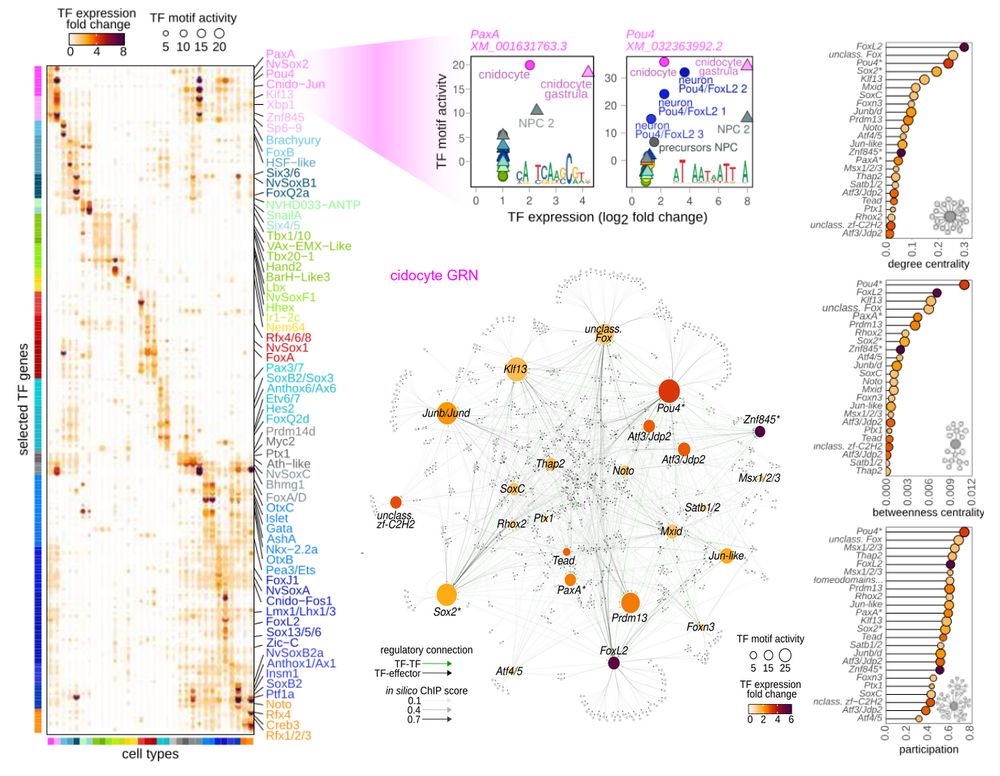
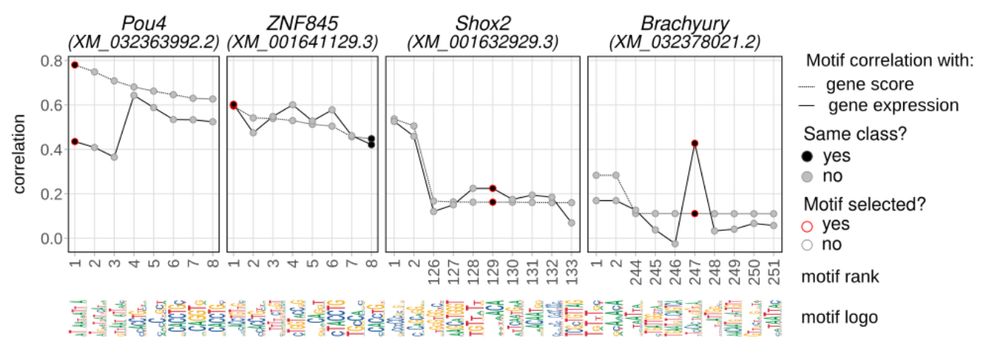
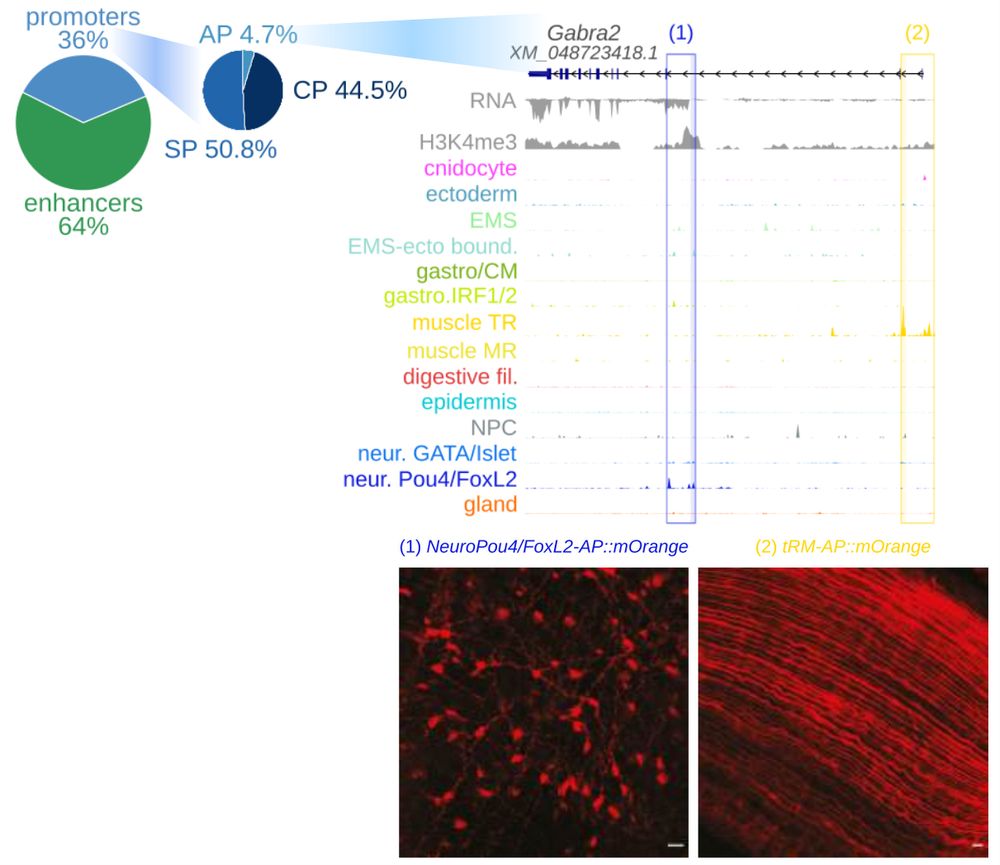
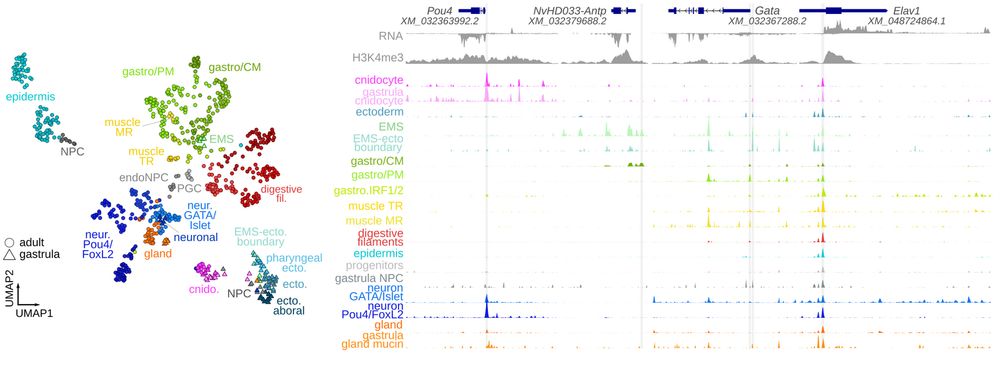
Animal cell types are defined by differential access to genomic information, a process orchestrated by the combinatorial activity of transcription factors that bind to cis -regulatory elements (CREs) to control gene expression. However, the regulatory logic and specific gene networks that define cell identities remain poorly resolved across the animal tree of life. As early-branching metazoans, cnidarians can offer insights into the early evolution of cell type-specific genome regulation. Here, we profiled chromatin accessibility in 60,000 cells from whole adults and gastrula-stage embryos of the sea anemone Nematostella vectensis. We identified 112,728 CREs and quantified their activity across cell types, revealing pervasive combinatorial enhancer usage and distinct promoter architectures. To decode the underlying regulatory grammar, we trained sequence-based models predicting CRE accessibility and used these models to infer ontogenetic relationships among cell types. By integrating sequence motifs, transcription factor expression, and CRE accessibility, we systematically reconstructed the gene regulatory networks that define cnidarian cell types. Our results reveal the regulatory complexity underlying cell differentiation in a morphologically simple animal and highlight conserved principles in animal gene regulation. This work provides a foundation for comparative regulatory genomics to understand the evolutionary emergence of animal cell type diversity. ### Competing Interest Statement The authors have declared no competing interest. European Research Council, https://ror.org/0472cxd90, ERC-StG 851647 Ministerio de Ciencia e Innovación, https://ror.org/05r0vyz12, PID2021-124757NB-I00, FPI Severo Ochoa PhD fellowship European Union, https://ror.org/019w4f821, Marie Skłodowska-Curie INTREPiD co-fund agreement 75442, Marie Skłodowska-Curie grant agreement 101031767
www.biorxiv.org
Anamaria Elek
@aelek.bsky.social
· Jul 5
Reposted by Anamaria Elek
Julia Rühle
@juruehle.bsky.social
· May 9
Lars Velten
@larsplus.bsky.social
· May 8
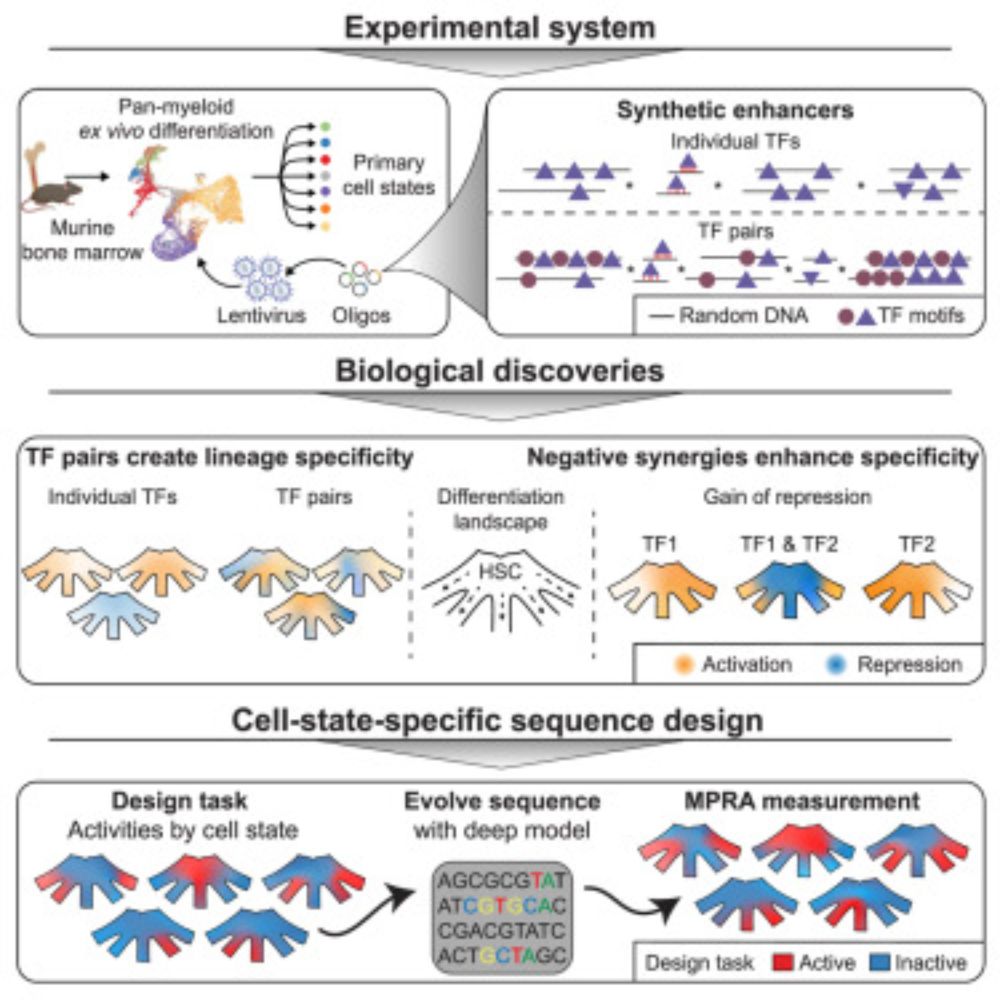
Design principles of cell-state-specific enhancers in hematopoiesis
Screen of minimalistic enhancers in blood progenitor cells demonstrates widespread
dual activator-repressor function of transcription factors (TFs) and enables the model-guided
design of cell-state-sp...
www.cell.com
Reposted by Anamaria Elek
Lars Velten
@larsplus.bsky.social
· May 8

Design principles of cell-state-specific enhancers in hematopoiesis
Screen of minimalistic enhancers in blood progenitor cells demonstrates widespread
dual activator-repressor function of transcription factors (TFs) and enables the model-guided
design of cell-state-sp...
www.cell.com
Reposted by Anamaria Elek
Iana V. Kim
@ianakim.bsky.social
· May 7
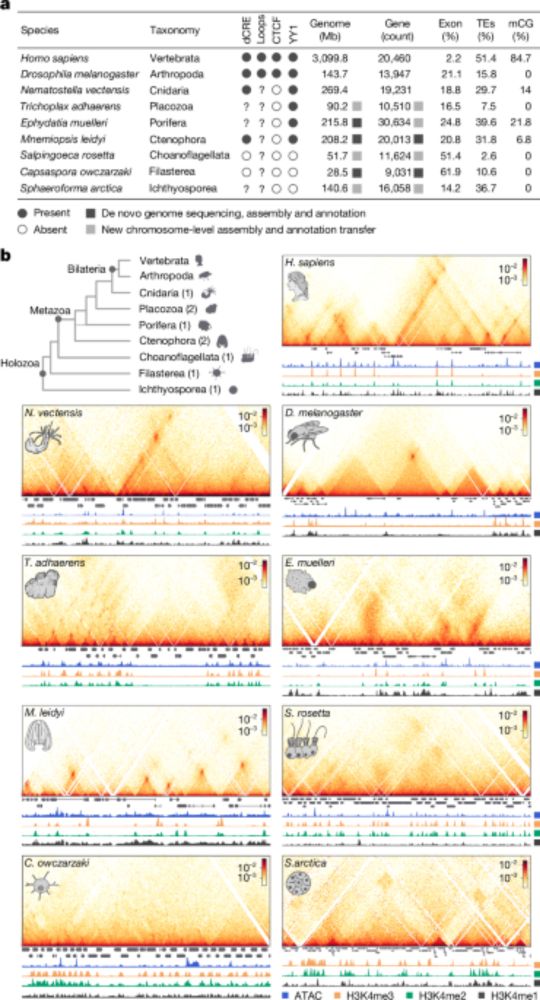
Chromatin loops are an ancestral hallmark of the animal regulatory genome - Nature
The physical organization of the genome in non-bilaterian animals and their closest unicellular relatives is characterized; comparative analysis shows chromatin looping is a conserved feature of ...
www.nature.com
Reposted by Anamaria Elek
Reposted by Anamaria Elek
Reposted by Anamaria Elek