Roland Roesler
@rolandroesler.bsky.social
630 followers
540 following
20 posts
Synthetic inorganic and organometallic chemistry at the University of Calgary.
Posts
Media
Videos
Starter Packs
Reposted by Roland Roesler
Reposted by Roland Roesler
Saurabh Chitnis
@sschitnis.bsky.social
· Apr 15

Geometrically Constrained Bismuth Compounds
A major thrust of research in modern main group chemistry is the use of multidentate ligands to perturb geometries at p-block elements such that they deviate substantially from valence shell electron-...
link.springer.com
Reposted by Roland Roesler
Roland Roesler
@rolandroesler.bsky.social
· Apr 10

Synthesis of a Transient Cationic Phosphaborene and its Trapping by Facile Aliphatic C−H Bond Activation
Abstraction of bromide from a B-brominated phosphaborene stabilized by a cyclic alkylamino carbene results in the formation of a transient cationic phosphaborene, which undergoes intramolecular C−H a....
doi.org
Reposted by Roland Roesler
Reposted by Roland Roesler
Reposted by Roland Roesler
Gilliard Group
@gilliardgroup.bsky.social
· Mar 25
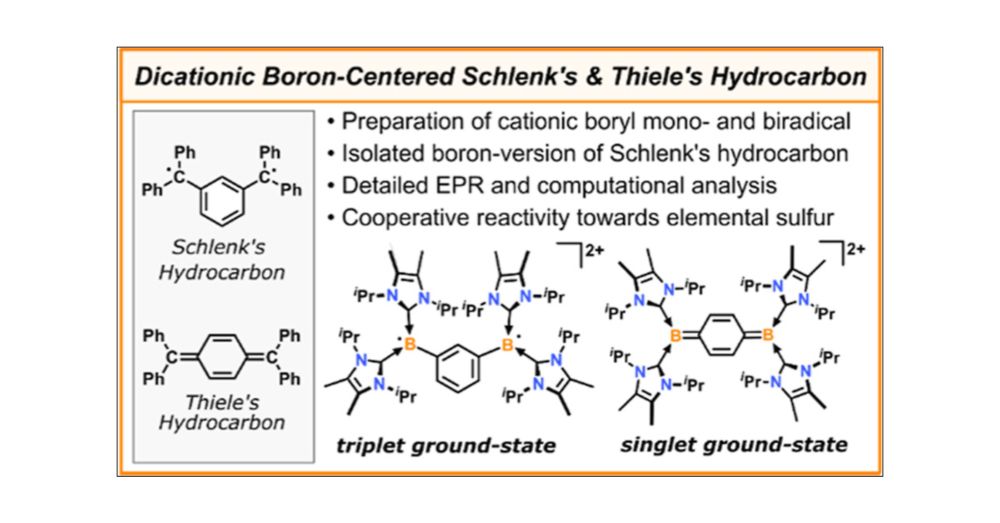
Dicationic Boron Derivatives of Schlenk’s and Thiele’s Hydrocarbon
In recent years, neutral NHC-stabilized boryl radicals have been investigated as reactive species in various organic transformations. However, cationic boron radicals have been significantly less explored. In addition, boron-centered open-shell species with S > 1/2 have emerged as attractive synthetic targets. In this study, we provide a synthetic route to an NHC-stabilized boryl radical cation as a salt of the weakly coordinating [Al(ORF)4]− (RF = C(CF3)3) anion. The synthetic procedure was extended to dicationic diboron derivatives of Schlenk’s and Thiele’s hydrocarbons with meta- and para-phenylene coupling units between the spin centers. While most known isolable boron biradicals have a singlet ground-state with a thermally accessible triplet state, the boron version of Schlenk’s hydrocarbon occupies a ground-state triplet spin-state, as shown by combined electron paramagnetic resonance spectroscopy and density functional theory studies. Furthermore, initial reactivity studies of the dications with elemental sulfur and diphenyldiselenide are presented.
pubs.acs.org
Roland Roesler
@rolandroesler.bsky.social
· Mar 30
Metal-free alkene hydroboration with pinacolborane employing C6F5BH2·SMe2 precatalyst
We have developed C6F5BH2·SMe2 as a unique, metal-free precatalyst for alkene hydroboration. It combines high reactivity and excellent regio- and chemoselectivity. Mechanistic studies reveal that the ...
pubs.rsc.org
Roland Roesler
@rolandroesler.bsky.social
· Mar 22

Synthesis of triple-decker sandwich compounds featuring a M–M bond through cyclo-Bi5 and cyclo-Sb5 rings - Nature Chemistry
Although several inorganic analogues of cyclopentadiene have been reported with group-15 elements, examples featuring the heavier elements Sb and Bi remain scarce. Now, a Bi5 ring has been stabilized ...
www.nature.com
Reposted by Roland Roesler
Reposted by Roland Roesler
Malte Fischer
@maltefischer.bsky.social
· Mar 19
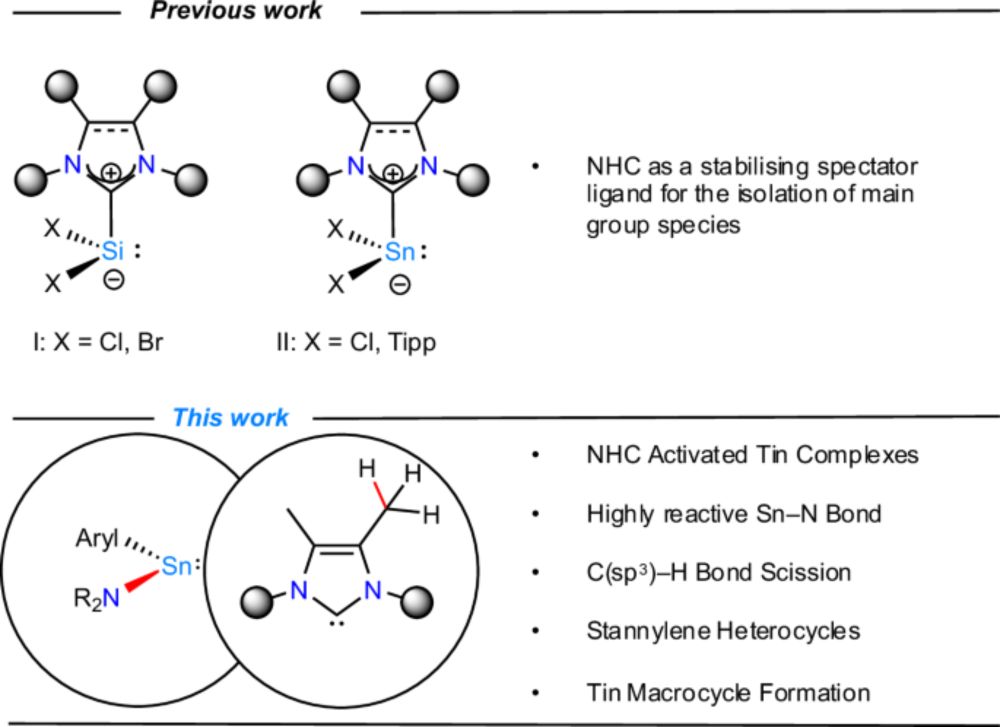
Carbene-activated stannylenes to access selective C(sp3)–H bond scission at the steric limit - Nature Communications
The ubiquity of N-heterocyclic carbenes (NHCs) in chemical research typically arises from their potent stabilizing capabilities and role as innocent spectators to stabilize otherwise non-bottleable co...
www.nature.com
Reposted by Roland Roesler
Marissa Clapson
@chemrissa.bsky.social
· Mar 18
Assistant Professor- Tenure Track Position- Department of Island Studies/Cleantech- Faculty of Arts *Amended closing date* | University of Prince Edward Island
The University of Prince Edward Island offers an amazing and affordable student experience in beautiful Charlottetown, PEI. Our interesting and unique programs bring out your best, lead you to realize...
www.upei.ca
Reposted by Roland Roesler
Jake Yeston
@jakeyeston.bsky.social
· Mar 13

Excited-state configuration of nitroarenes enables oxidative cleavage of aromatics over alkenes
The ozonolytic deconstruction of aromatics remains a challenge in organic chemistry. Ozone preferentially reacts with alkenes over arenes, meaning that once the initial aromatic cleavage occurs, the d...
www.science.org
Reposted by Roland Roesler
Reposted by Roland Roesler
Reposted by Roland Roesler
Phil Gale
@profphilgale.bsky.social
· Feb 17
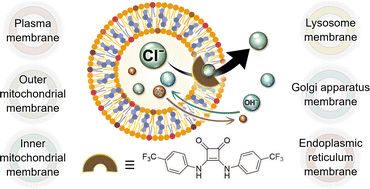
Anion transport in biologically relevant lipid mixtures
A potent squaramide-based anion transporter was used to evaluate how vesicles prepared with tailored lipid compositions, which mimic organelle membranes, can impact transmembrane transport. Using HPTS...
pubs.rsc.org
Reposted by Roland Roesler
Saurabh Chitnis
@sschitnis.bsky.social
· Feb 26
Recent advances in heavier Group 15 (P, As, Sb, Bi) radical chemistry – frameworks, small molecule reactivity, and catalysis
Main group radical chemistry has been a targeted research area for several decades. With growing examples of phosphorus radicals, heavier pnictogen radicals including arsenic, antimony, and bismuth ha...
pubs.rsc.org
Reposted by Roland Roesler
Neal Mankad
@metallacycle.bsky.social
· Feb 24

Comprehensive Re-examination of Intermetallic Charge Density for the Metal Carbonyl Dimers Mn2(CO)10 and [(η5-C5H5)Fe(CO)2]2
In this study, we have employed high-resolution charge density (HRCD) techniques using X-ray diffraction data collected at 10 K to reanalyze the intermetallic electron density topologies of two repres...
pubs.acs.org