Melanie McDowell
@melaniemcdowell.bsky.social
660 followers
350 following
12 posts
Group Leader at MPI of Biophysics exploring ER membrane protein biogenesis
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Melanie McDowell
Reposted by Melanie McDowell
Reposted by Melanie McDowell
Carolin Klose
@carolinklose.bsky.social
· Aug 11

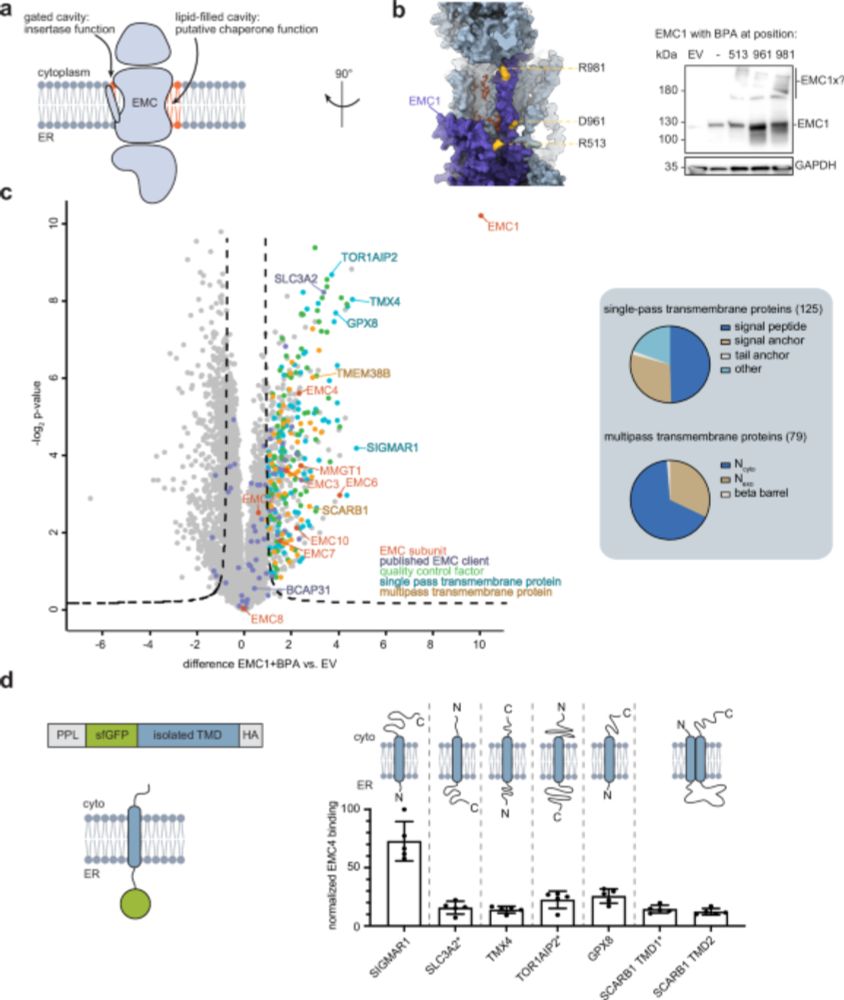
Structural basis of an EMC:Spf1 insertase-dislocase complex in the eukaryotic endoplasmic reticulum
Most eukaryotic membrane proteins are inserted into the membrane at the endoplasmic reticulum (ER). This essential but error-prone process relies on molecular quality control machineries to prevent mi...
www.biorxiv.org
Reposted by Melanie McDowell
Reposted by Melanie McDowell
Kedrov Lab @ HHU
@kedrov-lab.bsky.social
· May 27

Substrate-induced assembly and functional mechanism of the bacterial membrane protein insertase SecYEG-YidC
The universally conserved Sec translocon and the YidC/Oxa1-type insertases mediate biogenesis of alpha-helical membrane proteins, but the molecular basis of their cooperation has remained disputed over decades. A recent discovery of a multi-subunit insertase in eukaryotes has raised the question about the architecture of the putative bacterial ortholog SecYEG-YidC and its functional mechanism. Here, we combine cryogenic electron microscopy with cell-free protein synthesis in nanodiscs to visualize biogenesis of the polytopic membrane protein NuoK, the subunit K of NADH-quinone oxidoreductase, that requires both SecYEG and YidC for insertion. We demonstrate that YidC is recruited to the back of the translocon at the late stage of the substrate insertion, in resemblance to the eukaryotic system, and in vivo experiments indicate that the complex assembly is vital for the cells. The nascent chain does not utilize the lateral gate of SecYEG, but enters the lipid membrane at the SecYE-YidC interface, with YidC being the primary insertase. SecYEG-YidC complex promotes folding of the nascent helices at the interface prior their insertion, so the examined cellular pathway follows the fundamental thermodynamic principles of membrane protein folding. Our data provide the first detailed insight on the elusive insertase machinery in the physiologically relevant environment, highlight the importance of the nascent chain for its assembly, and prove the evolutionary conservation of the gate-independent insertion route. ### Competing Interest Statement The authors have declared no competing interest. Deutsche Forschungsgemeinschaft, https://ror.org/018mejw64, Ke1879/3, 267205415 (CRC 1208) European Research Council, https://ror.org/0472cxd90, CRYOTRANSLATION
www.biorxiv.org
Reposted by Melanie McDowell
Reposted by Melanie McDowell