Reposted by: Shaun Treweek
trialsjournal.biomedcentral.com/articles/10....

trialsjournal.biomedcentral.com/articles/10....
@ukcrc-ctu.org.uk @ecrin.bsky.social

Thanks to everyone – research participants, patients, professionals, volunteers and regulatory bodies – working together to do health and social care research.
rdforum.nhs.uk/red4research...
#Red4Research

trialsjournal.biomedcentral.com/articles/10....
trialsjournal.biomedcentral.com/articles/10....

Reposted by: Shaun Treweek
➡️ If you're joining The People's Review for the first time sign-up here: buff.ly/kAuYITA
➡️ If joined us for Stage 1 you can login in directly to Cochrane Crowd here: buff.ly/q0WudTM
clinical-research-facility.ed.ac.uk/edinburgh-cl...

💥 That’s where #ThePeoplesReview comes in. Learn about systematic reviews, by doing a systematic review
🔗https://www.thepeoplesreview.ie/join-now

Reposted by: Shaun Treweek
www.jclinepi.com/article/S089...
Reposted by: Shaun Treweek
🗓️ 2 June
🕙 10:00-11:30 BST
📍 Online
Register now: bit.ly/4k28ALd

Reposted by: Shaun Treweek

💥 That’s where #ThePeoplesReview comes in. Learn about systematic reviews, by doing a systematic review
www.thepeoplesreview.ie

Reposted by: Shaun Treweek, Marion Campbell
📌 CONSORT 2025 replaces all previous versions and should be used from now on.
So what’s new and what’s different? 1/7
#MethodologyMonday #116
(COI - I am a co-author)
Reposted by: Shaun Treweek
#MethodologyMonday
Scott & Westwell
orca.cardiff.ac.uk/id/eprint/17...
Reposted by: Shaun Treweek
Full article here: researchinvolvement.biomedcentral.com/articles/10....
#ThePeoplesReview #PoweredByThePublic #ArticleOfTheMonth
Reposted by: Shaun Treweek
Check out:
www.trialforge.org/excelsior-pi...
The is part of EXCELSIOR, a project ed by Frances Shiely at Cork University.
Reposted by: Shaun Treweek
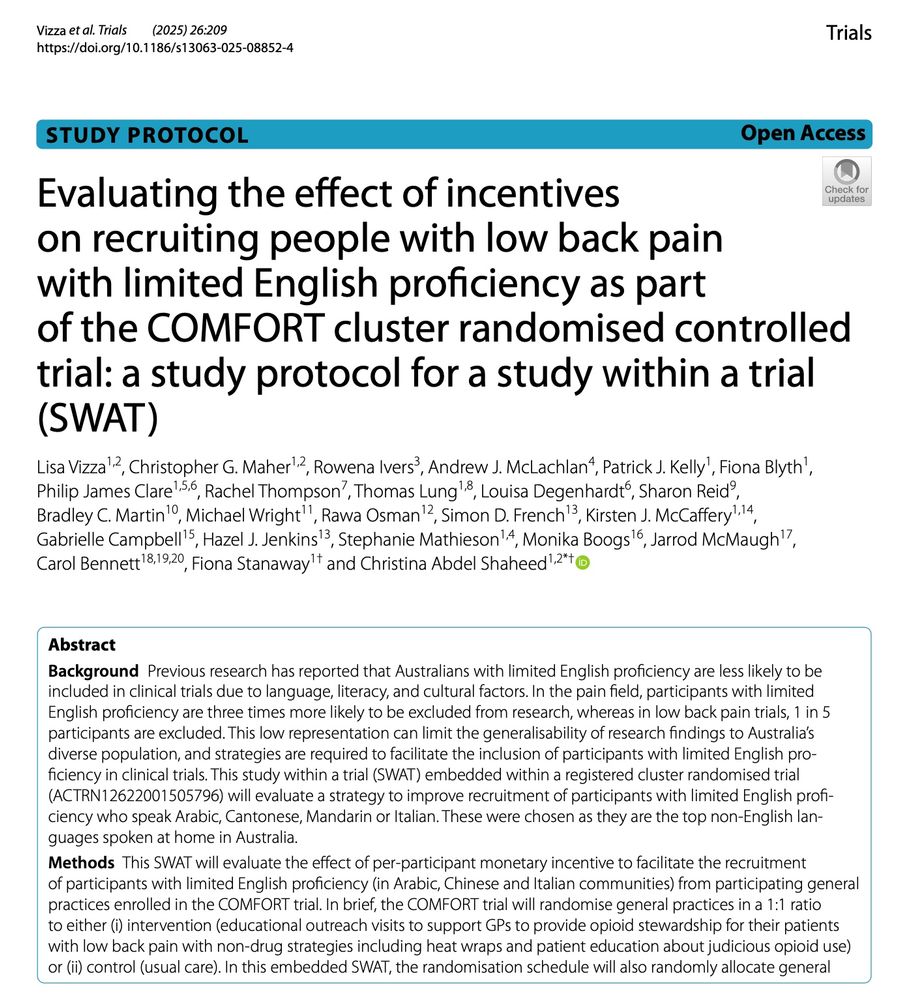
In their follow-up study in ‘Tolerating bad health research’, Shaun Treweek and colleagues conducted risk of bias assessment of 140 UK, Irish and Canadian trials. Despite the bad trials (55%), trials judged to be good increased from 9% to 16%.
trialsjournal.biomedcentral.com/articles/10....

Reposted by: Shaun Treweek


trialsjournal.biomedcentral.com/articles/10....
In summary, after risk of bias reassessment of 140 UK, Irish and Canadian trials, there were still as many bad trials (55%) but trials judged to be good increased from 9% to 16%.
Not very reassuring really.

by The Lancet — Reposted by: Shaun Treweek
In our latest issue, a recent trial evaluates the impact of two behavioural interventions on uptake of FIT colorectal screening.
Find this & more 👉 tinyurl.com/y9sjj4hc
![Cover of the March 29, 2025 issue of The Lancet. Quote on cover reads: “Our findings show the potential for low-cost or no-cost behavioural interventions to increase participation in screening and reduce deaths from colorectal cancer [by] adding one sentence to the invitation letter.”](https://cdn.bsky.app/img/feed_thumbnail/plain/did:plc:ypu6bwefbbzhcvojdj7mz657/bafkreiagkucwtre7ywn3o4sbds7loqvbyrtnjuysv2bigcd4lxkzzn5jkq@jpeg)
www.bmj.com/content/388/...
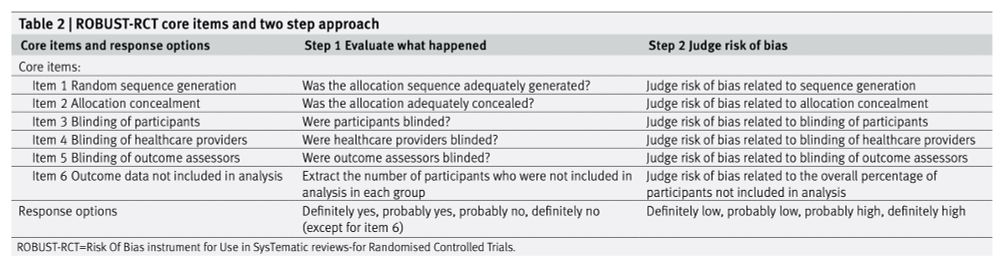
Similar to an older Cochrane risk of bias tool, it looks like something that could actually be used, which is great. The team explicitly aimed to balance simplicity and methodological rigour.
Reposted by: Shaun Treweek
www.academia.edu/2998-7741/1/...
Reposted by: Shaun Treweek
Audit and feedback: effects on professional practice - Ivers, N - 2025 www.cochranelibrary.com/cdsr/doi/10....

I had all the bits ready bar two sentences and the short cover letter. It still took 1 hour 49 mins. Bonkers. Do they really need all that info at submission?
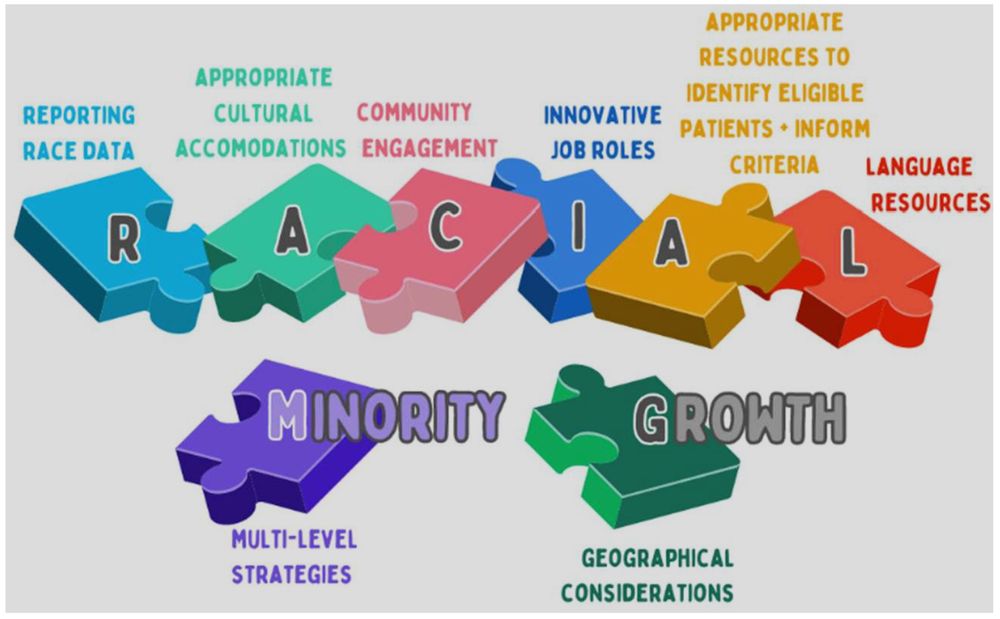
In summary, very few countries reported the involvement of ethnic minority community members in research priority-setting.
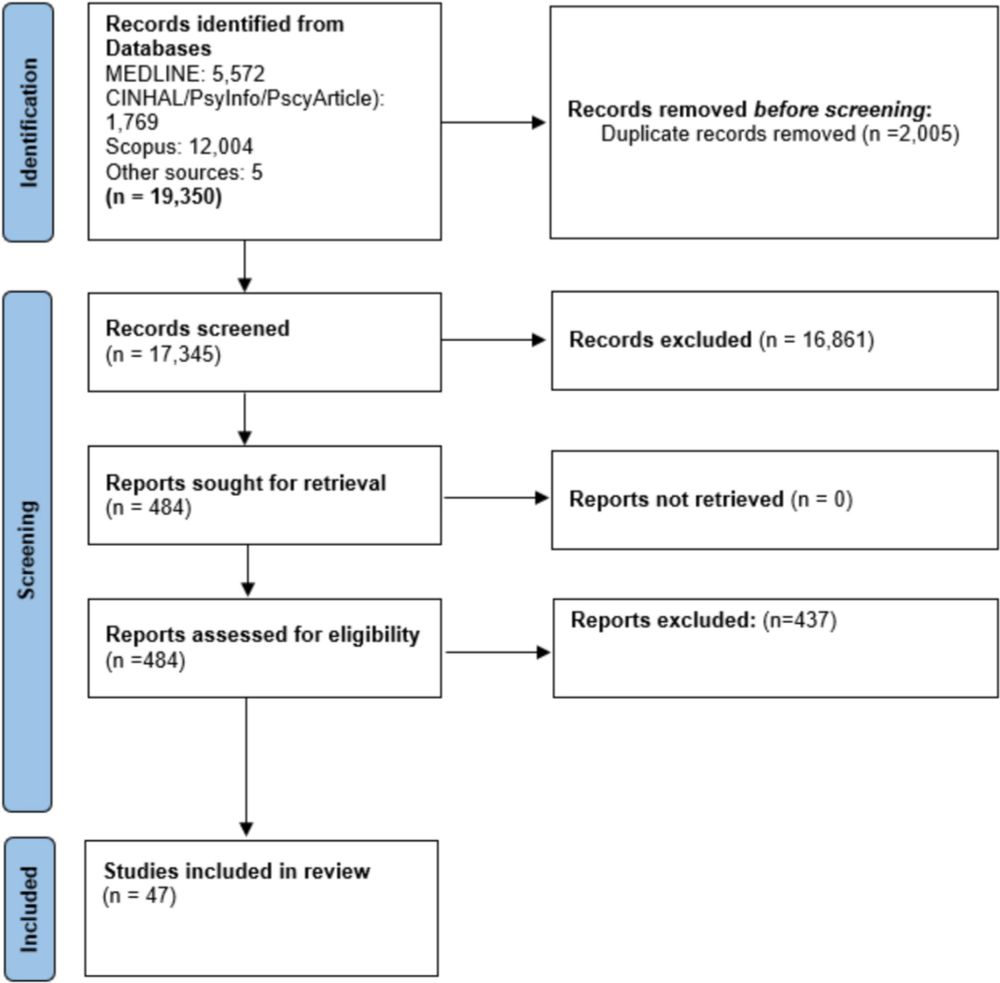
link.springer.com/article/10.1...
Reposted by: Shaun Treweek

Reposted by: Shaun Treweek

Reposted by: Shaun Treweek
Please consider taking part in a Aberdeen-led survey that explores the practices of sharing recruitment and/ or retention rates with recruiting sites as a method to improve recruitment and/or retention behaviours.
forms.office.com/e/9RCxiiCXM2.