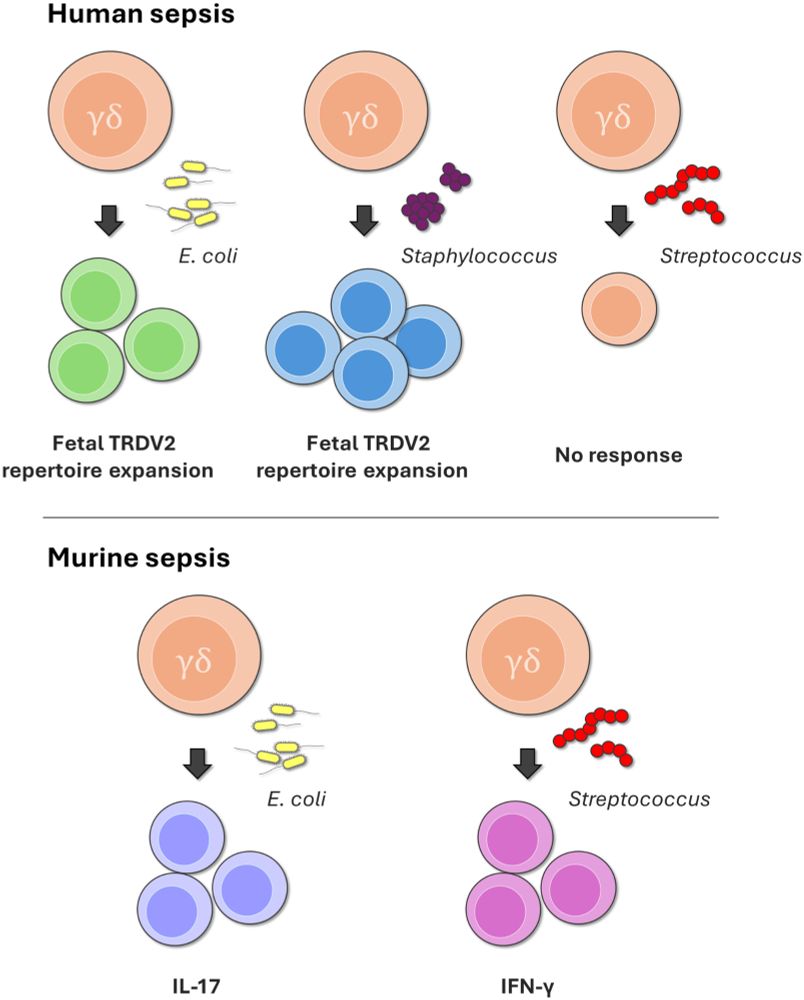
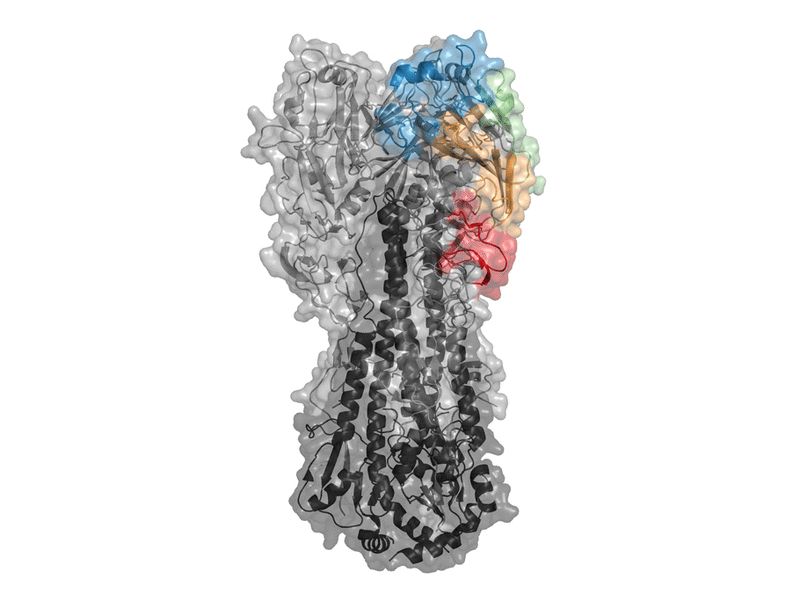
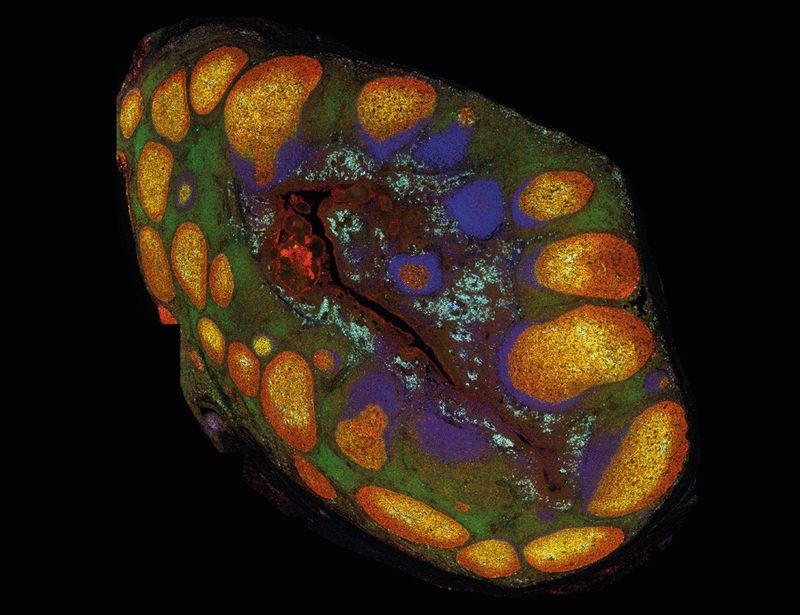
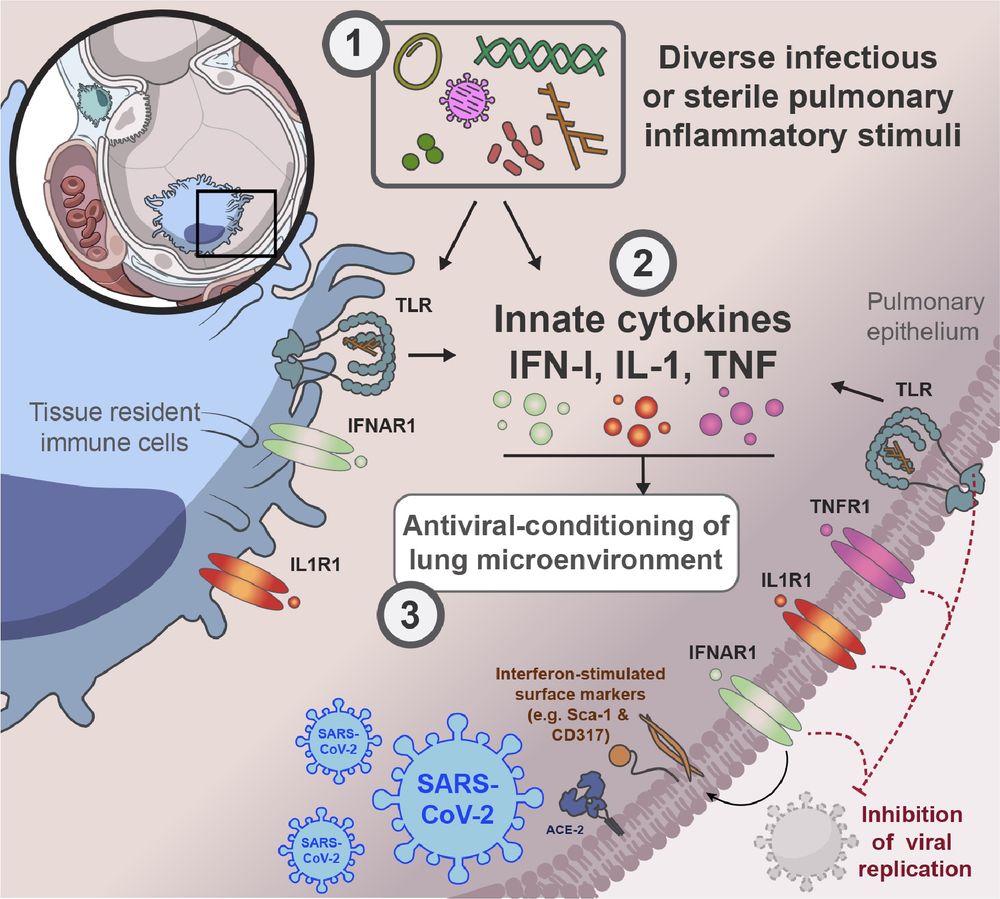
Hannah Isles
@hannah-isles.bsky.social
1.5K followers
270 following
10 posts
Associate editor at Science Immunology
Swiftie ✌🏻
Sheffield, UK 🌳
Posts
Media
Videos
Starter Packs
Hannah Isles
@hannah-isles.bsky.social
· Mar 12
Hannah Isles
@hannah-isles.bsky.social
· Mar 12
Reposted by Hannah Isles
Claire Olingy
@claireolingy.bsky.social
· Jan 27
Hannah Isles
@hannah-isles.bsky.social
· Jan 27
Reposted by Hannah Isles
Reposted by Hannah Isles
Reposted by Hannah Isles
Hannah Isles
@hannah-isles.bsky.social
· Nov 13
Reposted by Hannah Isles
Carolyn King
@immunologyking.bsky.social
· Nov 13