Mariko Sasaki
@marikosasakiatnig.bsky.social
40 followers
33 following
2 posts
Tenure track PI at NIG in Japan, studying extrachromosomal circular DNA (ecDNA) in cancer, eccDNA biology, ERC, DNA copy number changes
Posts
Media
Videos
Starter Packs
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Dirk Remus
@dirkremus.bsky.social
· Mar 6
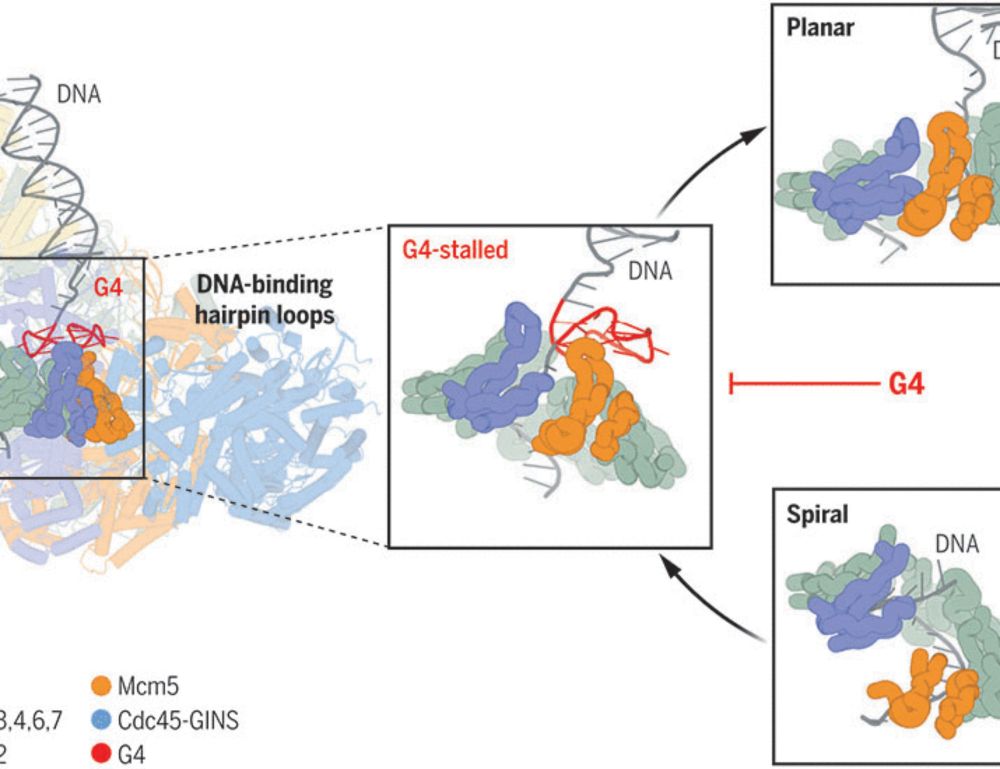
G-quadruplex–stalled eukaryotic replisome structure reveals helical inchworm DNA translocation
DNA G-quadruplexes (G4s) are non–B-form DNA secondary structures that threaten genome stability by impeding DNA replication. To elucidate how G4s induce replication fork arrest, we characterized fork ...
www.science.org
Reposted by Mariko Sasaki
The Groth lab
@grothlab.bsky.social
· Feb 19
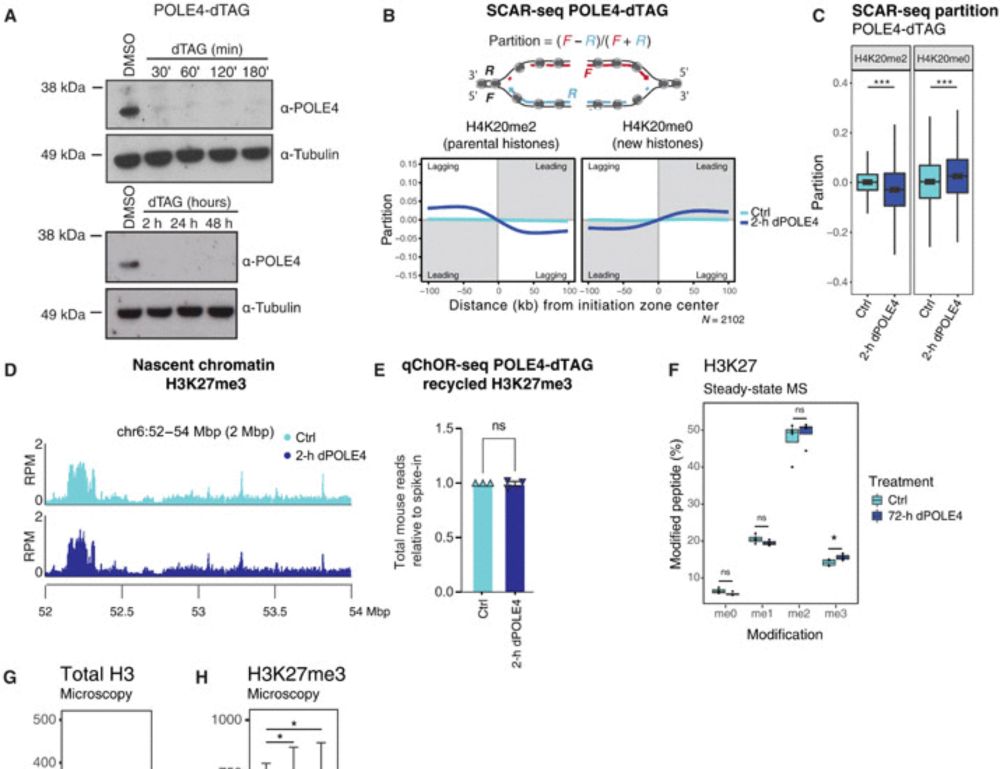
Disabling leading and lagging strand histone transmission results in parental histones loss and reduced cell plasticity and viability
Losing parental histones during DNA replication fork passage challenges differentiation competence and cell viability.
tinyurl.com
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Di Jiang
@dijiang319.bsky.social
· Feb 8
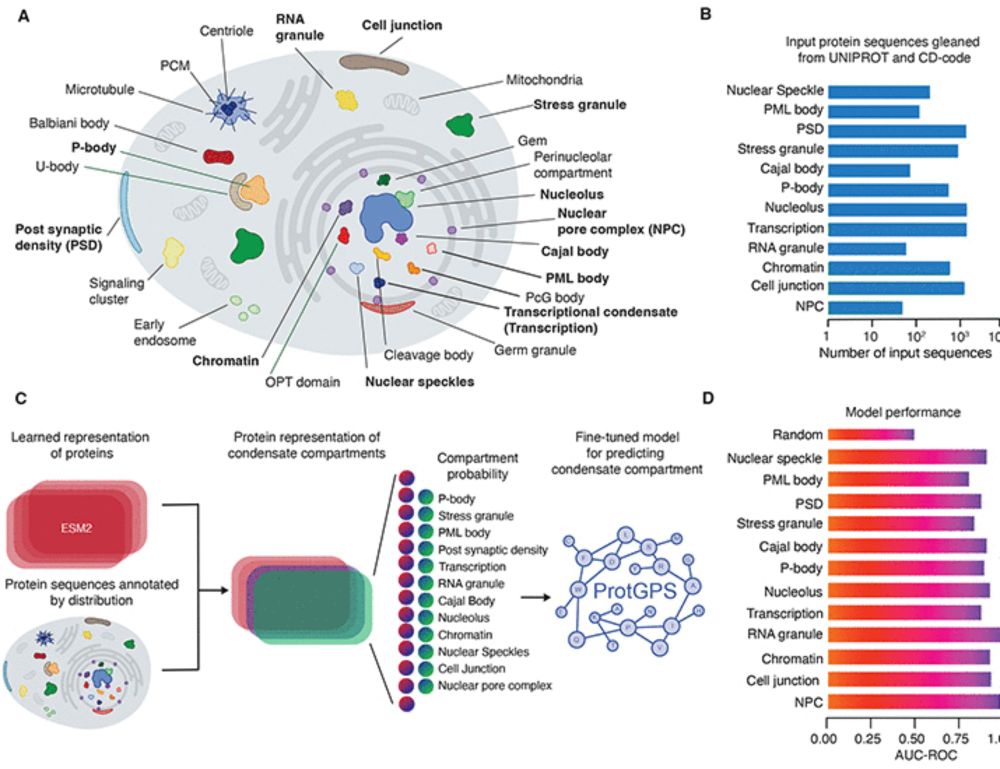
Protein codes promote selective subcellular compartmentalization
Cells have evolved mechanisms to distribute ~10 billion protein molecules to subcellular compartments where diverse proteins involved in shared functions must assemble. Here, we demonstrate that prote...
www.science.org
Reposted by Mariko Sasaki
Takashi Fukaya
@fukayalab.bsky.social
· Feb 3

Postdoctoral Researcher in chromosome organization and function
Do you want to contribute to top quality medical research? Join the lab of Camilla Björkegren at the Department of Cell and Molecular Biology, Karolinska Institutet, Stockholm, Sweden, to study molec
ki.varbi.com
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Reposted by Mariko Sasaki
Anton Henssen
@anton-henssen.bsky.social
· Jan 31
Sudarshan Pinglay
@sudpinglay.bsky.social
· Jan 31
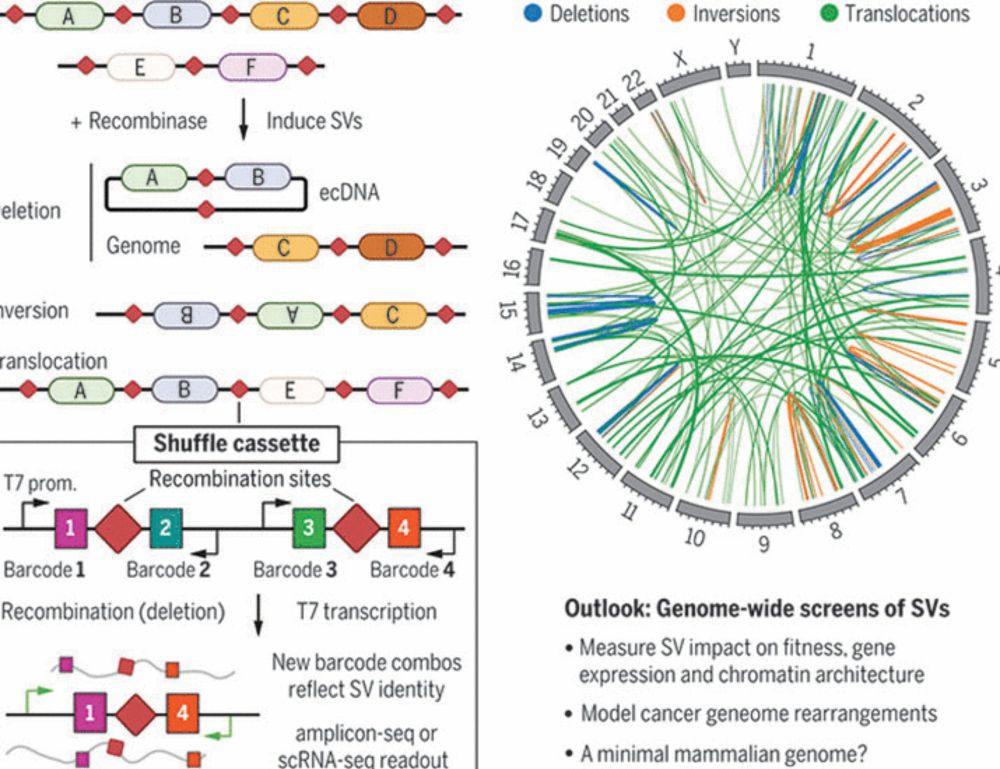
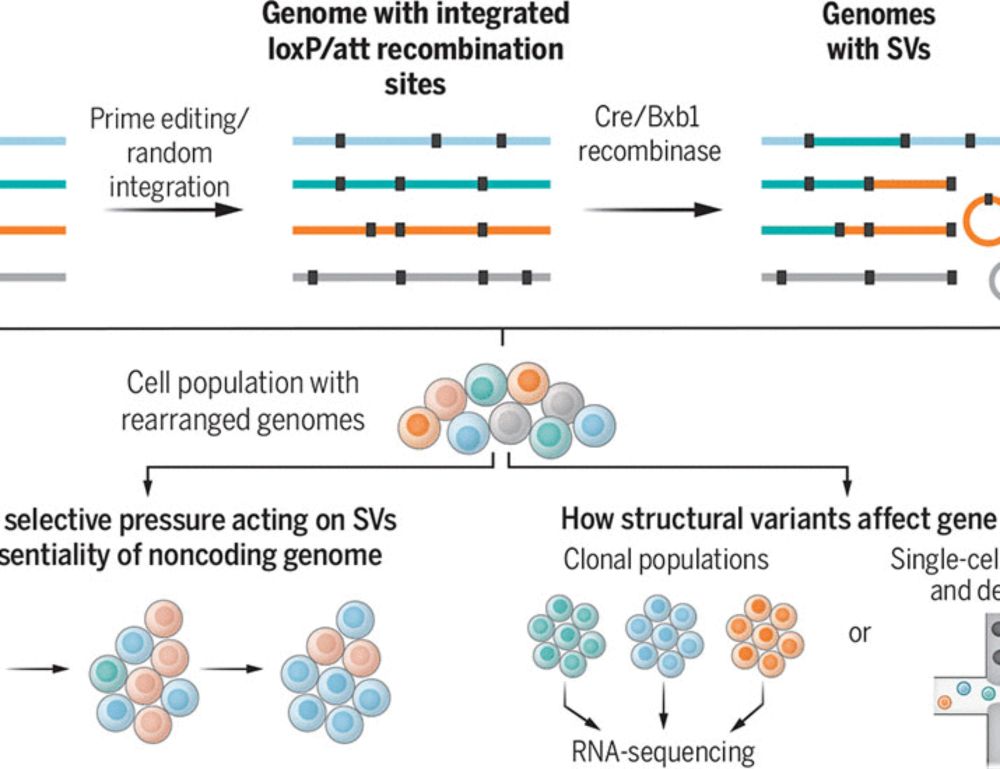
Multiplex generation and single-cell analysis of structural variants in mammalian genomes
Studying the functional consequences of structural variants (SVs) in mammalian genomes is challenging because (i) SVs arise much less commonly than single-nucleotide variants or small indels and (ii) ...
www.science.org
Reposted by Mariko Sasaki