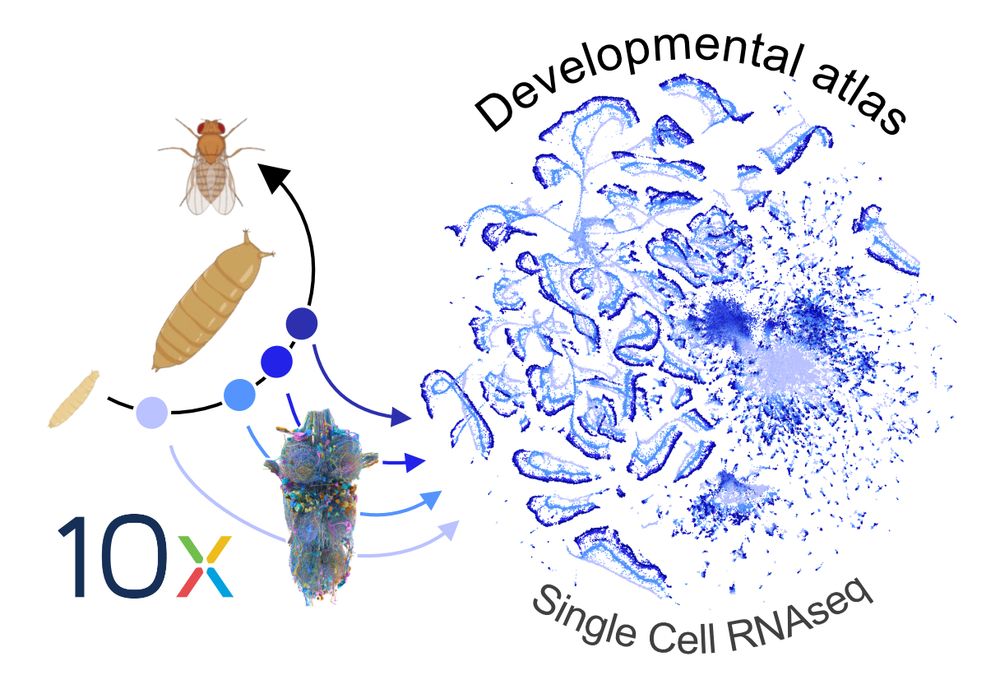
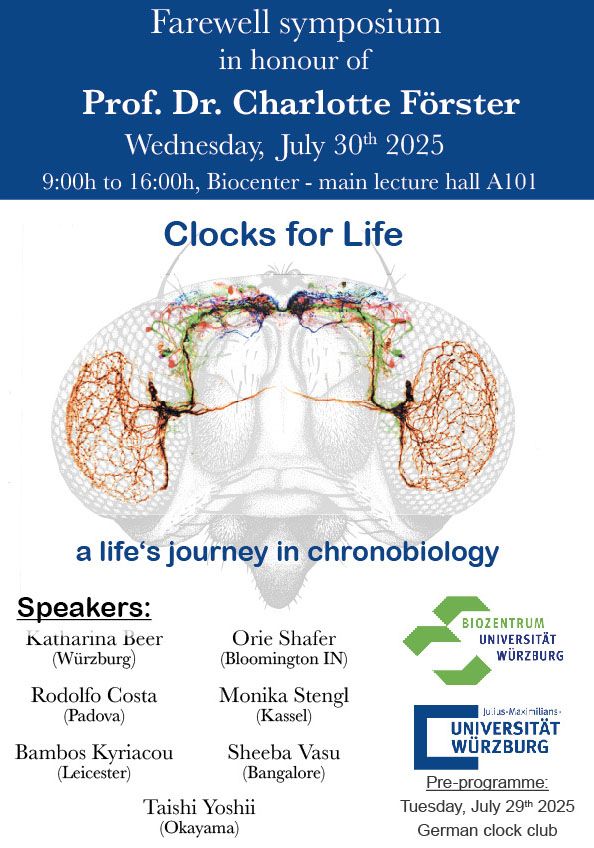
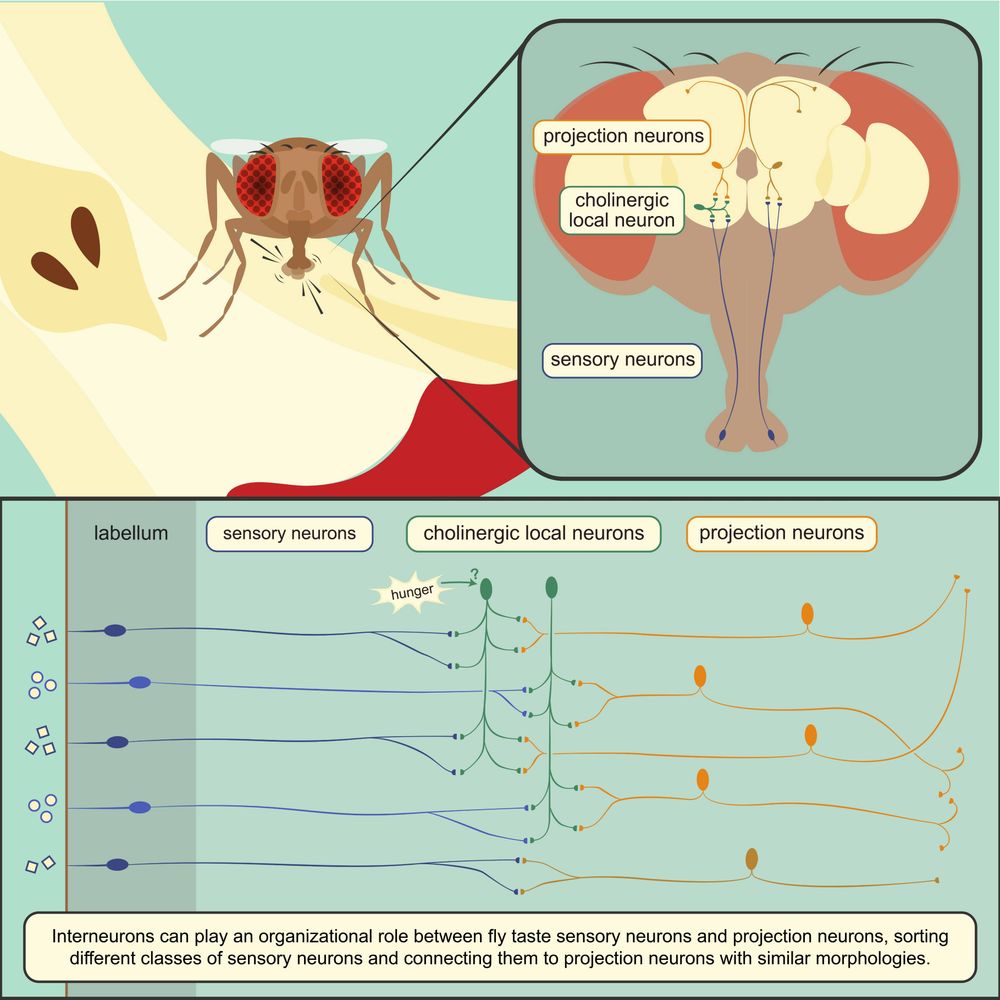
Meet Zandawala
@zandawala.bsky.social
300 followers
400 following
37 posts
Assistant Professor (University of Nevada Reno)
Group leader (University of Würzburg)
Interested in neuromodulation of Drosophila physiology and behavior
Posts
Media
Videos
Starter Packs
Meet Zandawala
@zandawala.bsky.social
· Aug 25
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Meet Zandawala
@zandawala.bsky.social
· Jun 28
Meet Zandawala
@zandawala.bsky.social
· Jun 28
Meet Zandawala
@zandawala.bsky.social
· Jun 27
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Eve Seuntjens
@eveseuntjens.bsky.social
· May 15

Postdoctoral researcher in evolutionary developmental neurobiology
Come join our EU cephalopod consortium to discover how octopuses and other coleoids developed their large nervous system. Postdoc position available in the Seuntjens Lab in Leuven, Belgium.
www.kuleuven.be
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Meet Zandawala
@zandawala.bsky.social
· May 6
Reposted by Meet Zandawala
Reposted by Meet Zandawala
Lukas Hüppe
@lhueppe.bsky.social
· May 5

A circadian clock drives behavioral activity in Antarctic krill (Euphausia superba) and provides a potential mechanism for seasonal timing
Behavioral experiments provide strong evidence that krill swimming behavior is controlled by biological clocks throughout the year, revealing a key mechanism for adaptation to high-latitude habitats.
doi.org
Reposted by Meet Zandawala
Luis Guerra
@luisguerra.bsky.social
· Apr 30
Reposted by Meet Zandawala
Luis Guerra
@luisguerra.bsky.social
· Apr 24

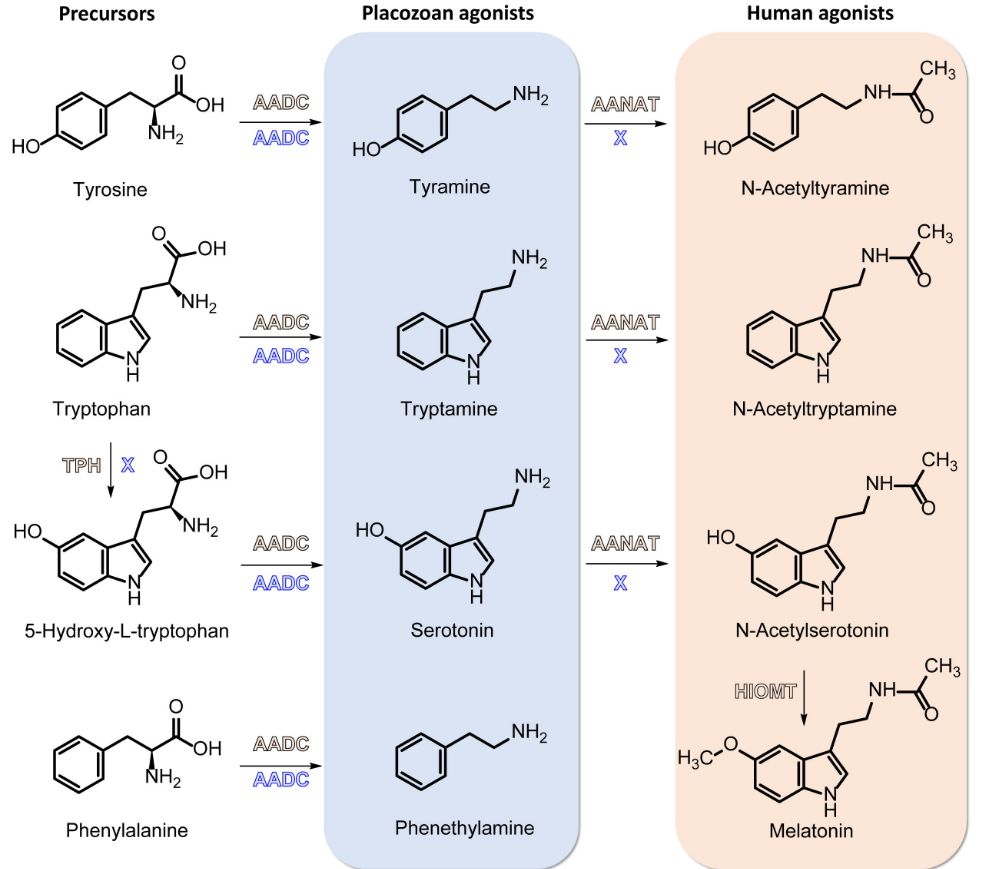
Functional and phylogenetic analysis of placozoan GPCRs reveal the prebilaterian origin of monoaminergic signalling.
Monoamines are biologically active compounds crucial for neurotransmission and various physiological processes. They include neurotransmitters like serotonin, dopamine, and melatonin, which regulate mood, movement, and sleep in humans. In ecdysozoans, monoamines such as tyramine are important for modulating locomotion, learning, and feeding. The monoaminergic signalling system has been considered a bilaterian innovation, with conflicting evidence supporting its existence in earlier branching, non-bilaterian animals. Here, we challenge the bilaterian origin hypothesis by combining large-scale receptor deorphanisation with phylogenetic analyses to identify monoamine receptors from the placozoan Trichoplax adhaerens. We demonstrate that these receptors are homologous to known bilaterian GPCRs, and behavioural assays demonstrate that monoamines like tyramine and tryptamine affect the speed of locomotion and body shape of this animal, respectively. These responses, together with the presence of biosynthetic enzymes for these molecules, reveal that monoaminergic signalling is both active and endogenous in placozoans. Our findings provide compelling evidence for a prebilaterian origin of monoaminergic systems, reshaping our understanding of early nervous system evolution. ### Competing Interest Statement The authors have declared no competing interest.
www.biorxiv.org
Reposted by Meet Zandawala
Piali Sengupta
@senguptalab.bsky.social
· Apr 18
Meet Zandawala
@zandawala.bsky.social
· Apr 14
Meet Zandawala
@zandawala.bsky.social
· Mar 30
Meet Zandawala
@zandawala.bsky.social
· Mar 28