Eine Studie konnte einen Vorteil gegenüber #mRNA-Impfstoffen bei d Immunogenität zeigen...

Eine Studie konnte einen Vorteil gegenüber #mRNA-Impfstoffen bei d Immunogenität zeigen...
12-month persistence of immune responses to self-amplifying mRNA COVID-19 vaccines: ARCT-154 versus BNT162b2 vaccine - The Lancet Infectious Diseases
www.thelancet.com/journals/lan...

12-month persistence of immune responses to self-amplifying mRNA COVID-19 vaccines: ARCT-154 versus BNT162b2 vaccine - The Lancet Infectious Diseases
www.thelancet.com/journals/lan...
Anyhoo, this is a great resource on the current state of #COVID #COVID19 vaccines, with links to trial registries and […]
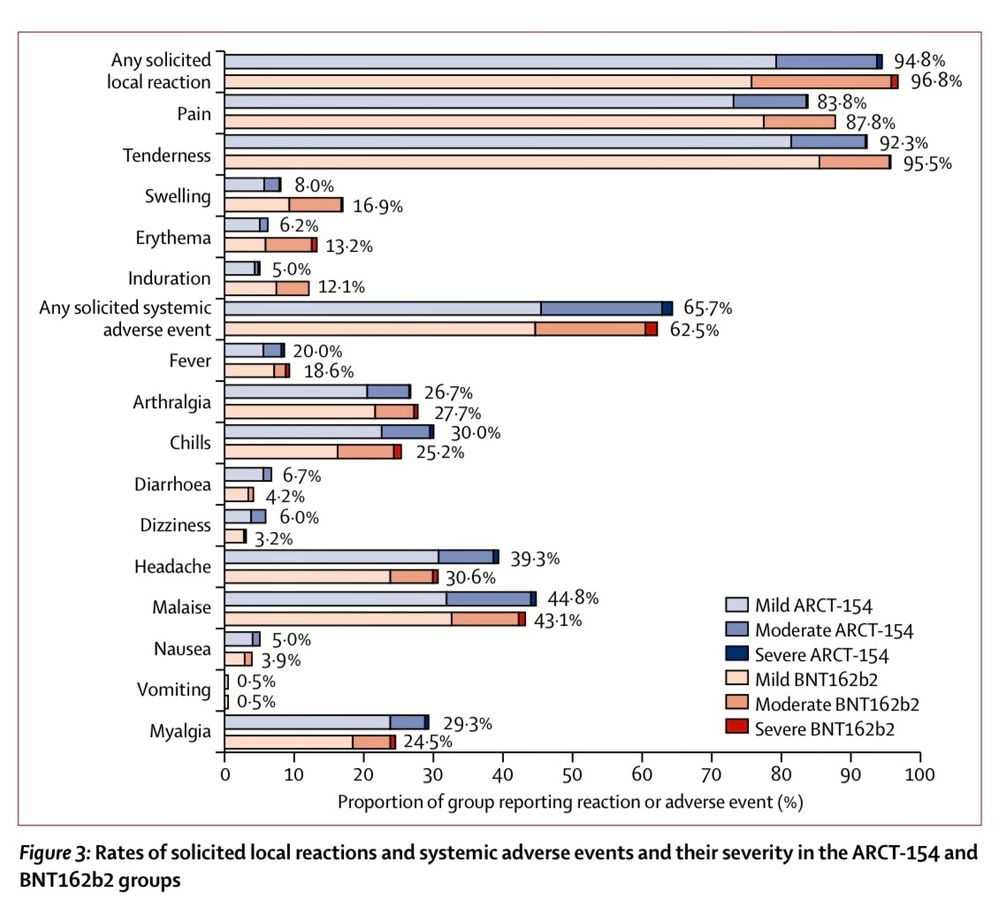
Booster dose of self-amplifying SARS-CoV-2 RNA vaccine vs. mRNA vaccine: a phase 3 comparison of ARCT-154 with Comirnaty®
www.medrxiv.org/cont...
2/3

Booster dose of self-amplifying SARS-CoV-2 RNA vaccine vs. mRNA vaccine: a phase 3 comparison of ARCT-154 with Comirnaty®
www.medrxiv.org/cont...
2/3
L’utilizzo della mascherina nei luoghi pubblici è diffusa. Polemiche non ce ne sono. Sul vaccino, fatto uno aggiornato (ARCT-154?), che sembra molto più efficace, su consiglio medico, lì.
Ps: si PAGA…
L’utilizzo della mascherina nei luoghi pubblici è diffusa. Polemiche non ce ne sono. Sul vaccino, fatto uno aggiornato (ARCT-154?), che sembra molto più efficace, su consiglio medico, lì.
Ps: si PAGA…
Safety profile of self-amplifying mRNA SARS-CoV-2 vaccine ARCT-154 in adults: a pooled phase 1/2/3 randomized clinical study. Data from trial with 17,000 show that ARCT-154 is safe and well tolerated.
pubmed.ncbi.nlm.nih.gov/40195167/ 5/5

Safety profile of self-amplifying mRNA SARS-CoV-2 vaccine ARCT-154 in adults: a pooled phase 1/2/3 randomized clinical study. Data from trial with 17,000 show that ARCT-154 is safe and well tolerated.
pubmed.ncbi.nlm.nih.gov/40195167/ 5/5
レプリコンワクチンの承認は日本で ARCT-154 が承認されているだけだと思ってたけど、既にインドで HGC019 (GEMCOVAC-19) が緊急使用承認されている事例があったのね。
考えられる安全性への懸念点として、免疫不全患者でクリアランスが不十分で残ってしまう可能性、妊婦での胎児への移行、ウイルスとの間の組み替えの発生など。
レプリコンワクチンの承認は日本で ARCT-154 が承認されているだけだと思ってたけど、既にインドで HGC019 (GEMCOVAC-19) が緊急使用承認されている事例があったのね。
考えられる安全性への懸念点として、免疫不全患者でクリアランスが不十分で残ってしまう可能性、妊婦での胎児への移行、ウイルスとの間の組み替えの発生など。
やはり Meiji Seika ファルマ(というか同列会社の明治製菓か?)への不買運動が効いているのかも知れないなと思った。またレプリコン・ワクチンに対する反対運動も。
新聞やTVを見ているだけでは、そういう事態に気がつかないのではないか。
私は反ワクチンではない。今まで新型コロナワクチンを6回打ってきた。しかし、今回のものに関しては保留である。
ja.wikipedia.org/wiki/ARCT-154
やはり Meiji Seika ファルマ(というか同列会社の明治製菓か?)への不買運動が効いているのかも知れないなと思った。またレプリコン・ワクチンに対する反対運動も。
新聞やTVを見ているだけでは、そういう事態に気がつかないのではないか。
私は反ワクチンではない。今まで新型コロナワクチンを6回打ってきた。しかし、今回のものに関しては保留である。
ja.wikipedia.org/wiki/ARCT-154
Dieser erste Nachweis der klinischen Wirksamkeit des sa-mRNA-Impfstoffs ARCT-154 gegen COVID-19 in Verbindung mit akzeptabler Sicherheit und Reaktogenität in einer großen 4/
Dieser erste Nachweis der klinischen Wirksamkeit des sa-mRNA-Impfstoffs ARCT-154 gegen COVID-19 in Verbindung mit akzeptabler Sicherheit und Reaktogenität in einer großen 4/
Gibt es den schon in Europa?🤔

Gibt es den schon in Europa?🤔
There is a higher level of antibodies, which may be sustained for longer.
2/4

There is a higher level of antibodies, which may be sustained for longer.
2/4

The follow-up from the in Japan-approved self-amplifying RNA vaccine
www.thelancet.com/jo...
1/4
The follow-up from the in Japan-approved self-amplifying RNA vaccine
www.thelancet.com/jo...
1/4
www.thelancet.com/journals/lan...
www.thelancet.com/journals/lan...
www.thelancet.com/journals/lan...

www.thelancet.com/journals/lan...
www.thelancet.com/journals/lan...
www.thelancet.com/journals/lan...



