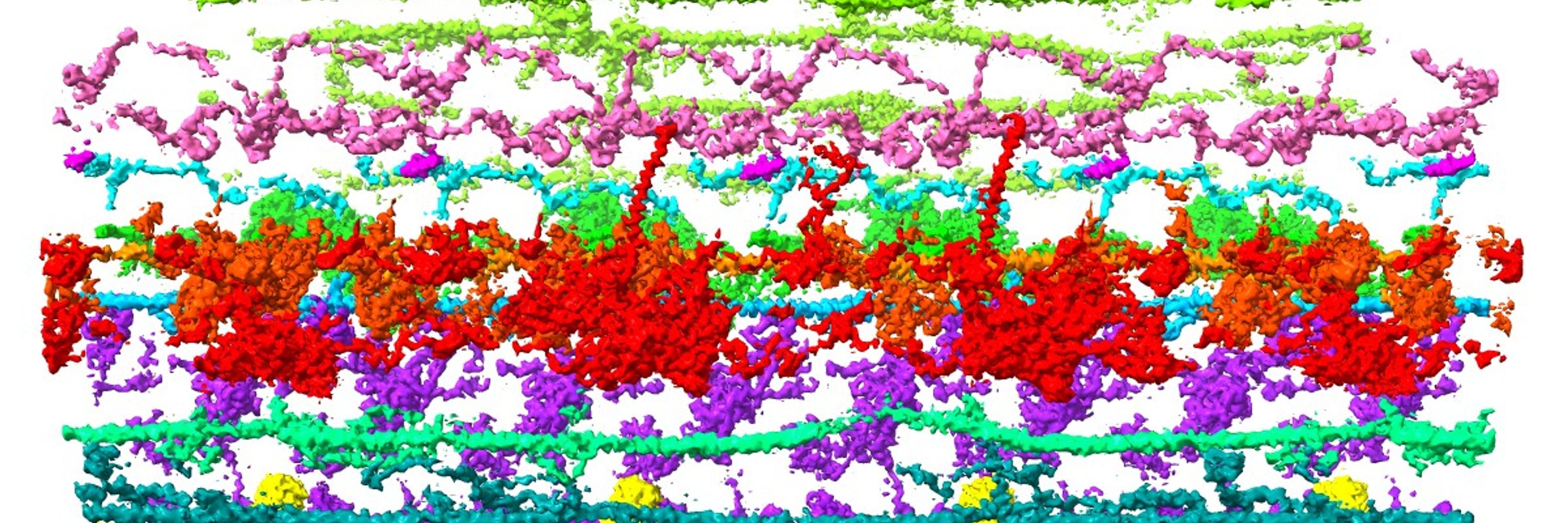
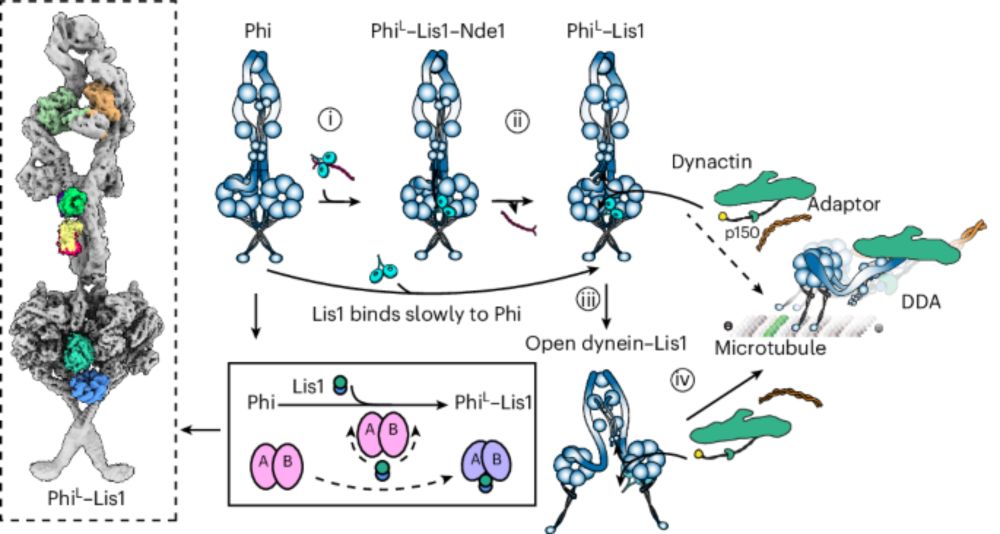
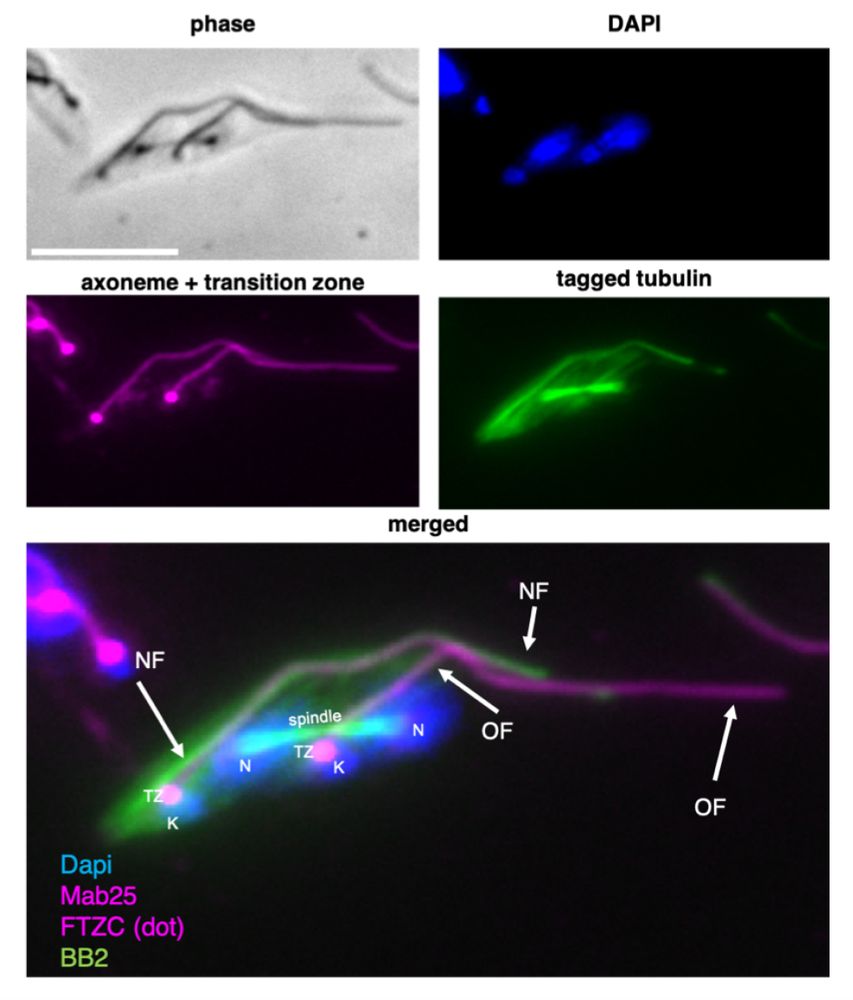
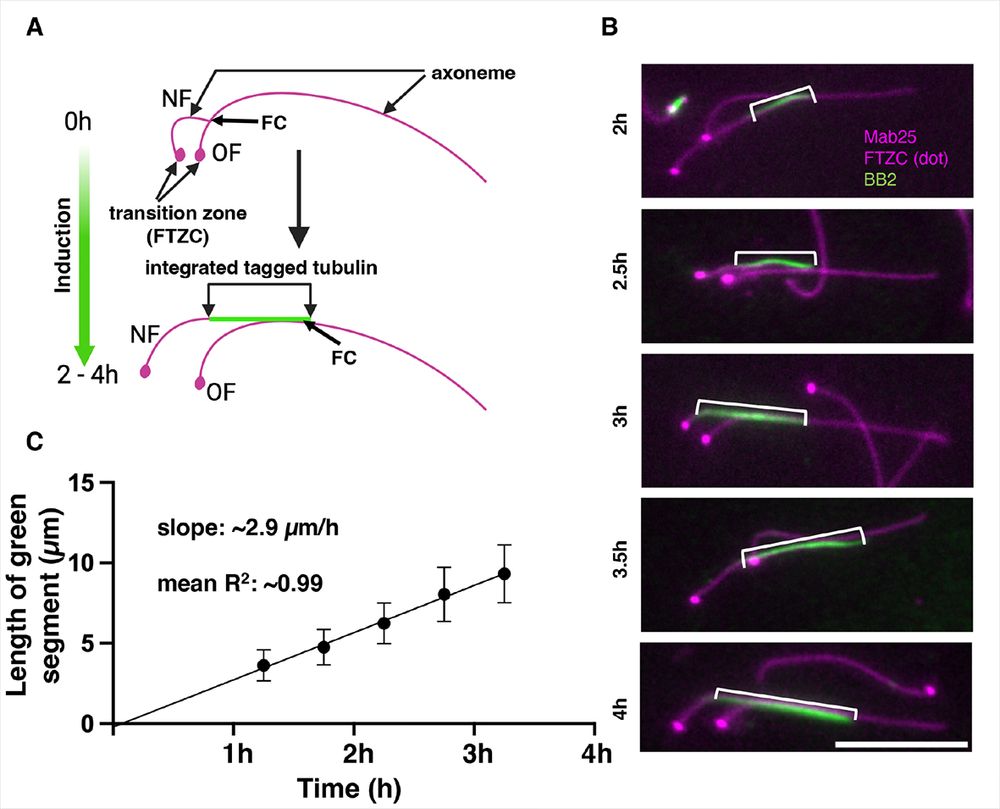
Yoshi Ichikawa
@ichikawa-lab.bsky.social
1.1K followers
1.6K following
77 posts
Tenure-track professor (PI) at Fudan University.
Have been working on motor protein, microtubule, and cilia.
Recently also working with membrane proteins.
Associate Editorial Board member of Cytoskeleton Journal.
Lab website: https://tinyurl.com/22c3eymy
Posts
Media
Videos
Starter Packs
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Rita Strack
@ritastrack.bsky.social
· Aug 1

Associate or Senior Editor, Nature Biomedical Engineering
Job Title: Associate or Senior Editor, Nature Biomedical Engineering Organization: Nature Portfolio Location: New York, Jersey City, Shanghai or Beijing – Hybrid Working Closing date: August 20, 2025 ...
springernature.wd3.myworkdayjobs.com
Reposted by Yoshi Ichikawa
Rita Strack
@ritastrack.bsky.social
· Aug 1

Associate Editor or Senior Editor, Nature Methods
Title: Associate or Senior Editor, Nature Methods Organization: Nature Portfolio Locations: New York, Jersey City, Shanghai or Beijing Closing Date: August 3, 2025 About Springer Nature Springer Na...
springernature.wd3.myworkdayjobs.com
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Shicheng Guo
@shihcheng.bsky.social
· Jun 4

Unveiling the structural spectrum of SARS-CoV-2 fusion by in situ cryo-ET | Nature Communications
SARS-CoV-2 entry into host cells is mediated by the spike protein, which drives membrane fusion. While cryo-EM reveals stable prefusion and postfusion conformations of the spike, the transient fusion intermediate states during the fusion process remain poorly understood. Here, we design a near-native viral fusion system that recapitulates SARS-CoV-2 entry and use cryo-electron tomography (cryo-ET) to capture fusion intermediates leading to complete fusion. The spike protein undergoes extensive structural rearrangements, progressing through extended, partially folded, and fully folded intermediates prior to fusion-pore formation, a process that depends on protease cleavage and is inhibited by the WS6 S2 antibody. Upon interaction with ACE2 receptor dimer, spikes cluster at membrane interfaces and following S2’ cleavage concurrently transition to postfusion conformations encircling the hemifusion and initial fusion pores in a distinct conical arrangement. S2’ cleavage is indispensable fo
doi.org
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Peter Andersen
@germline.bsky.social
· May 16

Tenure-Track Assistant Professor / Associate Professor in Bioinformatics at Aarhus University, Denmark - Vacancy at Aarhus University
Vacancy at Department of Molecular Biology and Genetics - BiRC - Bioinformatics Research Center, Aarhus University
mbg.au.dk
Reposted by Yoshi Ichikawa
Reposted by Yoshi Ichikawa
Jean Nakhle
@jeannakhle.bsky.social
· May 8