Andrew Deans
@genomestability.bsky.social
880 followers
490 following
1.1K posts
Head of Genome Stability Unit at SVI, Melbourne. All things DNA damage response: Fanconi Anaemia, Bloom Syndrome, Gene editing, R-loops, telomeres, HR & more
Posts
Media
Videos
Starter Packs
Pinned
Reposted by Andrew Deans
Mathew Jones
@jonesmjk.bsky.social
· Sep 1

A high-resolution, nanopore-based artificial intelligence assay for DNA replication stress in human cancer cells
Nature Communications - Determining how replication forks move across the human genome is critical for the effective use of agents that target replication stress. Here, the authors present...
rdcu.be
Reposted by Andrew Deans
Deborah Marsh
@djmarsh24.bsky.social
· Aug 20
Reposted by Andrew Deans
Basil Greber
@bjgreber.bsky.social
· Aug 15

High-throughput investigation of cyclin docking interactions reveals the complexity of motif binding determinants - Nature Communications
Many protein–protein interactions depend on Short Linear Motifs (SLiMs). In this study, the authors use large-scale binding assays, deep mutational scanning, and structural analysis to map SLiMs recog...
www.nature.com
Reposted by Andrew Deans
Reposted by Andrew Deans
Tony Cesare
@thecesarelab.bsky.social
· Aug 4
DNA-repair-driven cell death compels us to rethink cancer therapies - Nature Reviews Molecular Cell Biology
Emerging evidence suggests that, following genotoxic therapy, it is the repair of DNA double-strand breaks, rather than the damage itself, that frequently drives cancer cell death.
www.nature.com
Reposted by Andrew Deans
Reposted by Andrew Deans
Andrew Deans
@genomestability.bsky.social
· Jul 12
Reposted by Andrew Deans
ahellab.bsky.social
@ahellab.bsky.social
· Jul 10
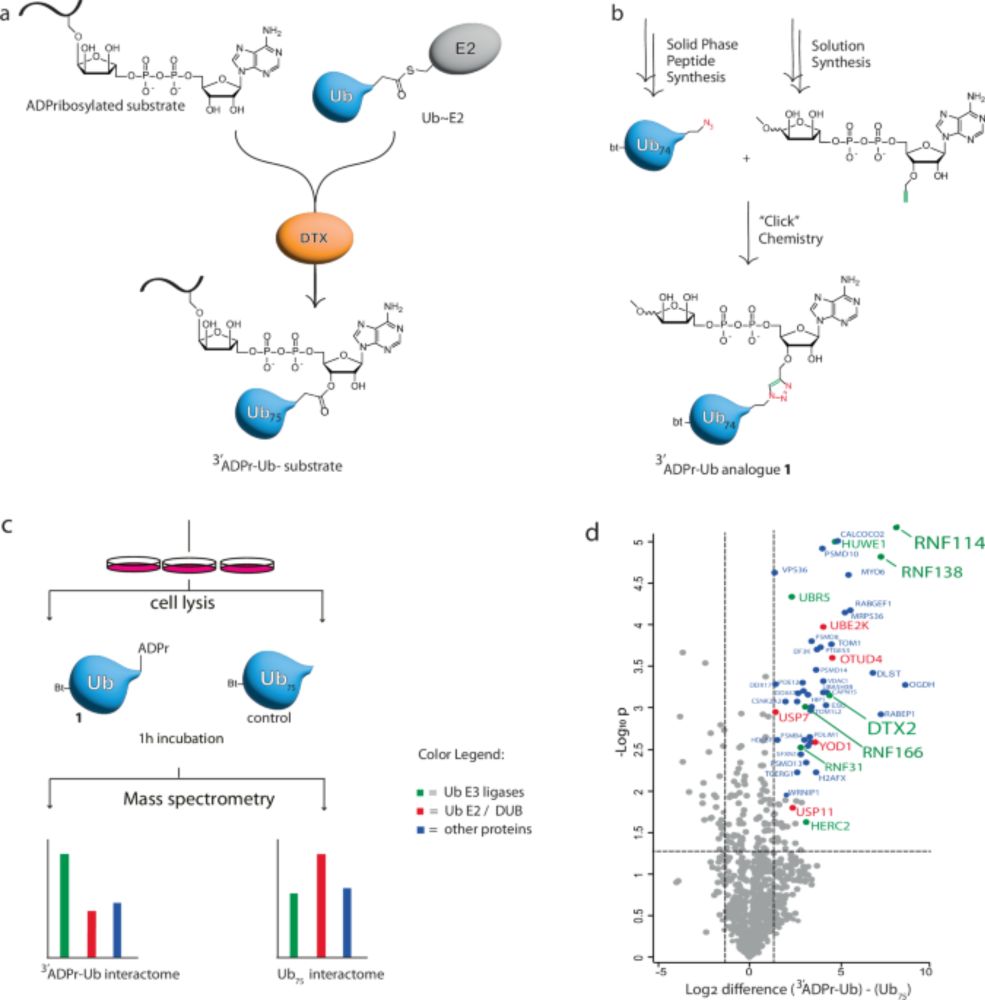
Identification of RNF114 as ADPr-Ub reader through non-hydrolysable ubiquitinated ADP-ribose - Nature Communications
Deltex E3s modify ADP-ribosylated targets with ubiquitin, creating a hybrid modification whose readers remains unknown. Here, the authors synthesise a non-hydrolysable probe that mimics the modificati...
www.nature.com
Reposted by Andrew Deans
NealeLab
@labneale.bsky.social
· Jul 6
Asymmetrical recognition and processing of double-strand breaks formed during DNA replication
DNA end resection to generate 3' ssDNA overhangs is the first step in homology-directed mechanisms of double-strand break (DSB) repair. While end resection has been extensively studied in the repair of endonuclease-induced DSBs, little is known about how resection proceeds at DSBs generated during DNA replication. We previously established a system to generate replication-dependent double-ended DSBs at the sites of nicks induced by the Cas9D10A nickase in the budding yeast genome. Here, we suggest that these DSBs form in an asymmetric manner, with one break end being blunt or near blunt, and the other bearing a 3' ssDNA overhang of up the size of an Okazaki fragment. We find that Mre11 preferentially binds blunt ends and is required for the removal Ku from these DSB ends. In contrast, the ends predicted to have 3' overhangs have minimal Ku binding, and end resection at these break ends can proceed in a mostly Mre11-independent manner through either the Exo1 or Dna2-Sgs1 long-range resection pathways. These findings indicate that resection proceeds differently at replication-dependent DSBs than at canonical DSBs, and reveals that Ku selectively binds nearly blunt ends, potentially explaining why replication-dependent DSBs are poorly repaired by non-homologous end joining. ### Competing Interest Statement The authors have declared no competing interest. National Institute of General Medical Sciences, https://ror.org/04q48ey07, R35GM126997
www.biorxiv.org
Reposted by Andrew Deans
Andrew Deans
@genomestability.bsky.social
· Jun 11
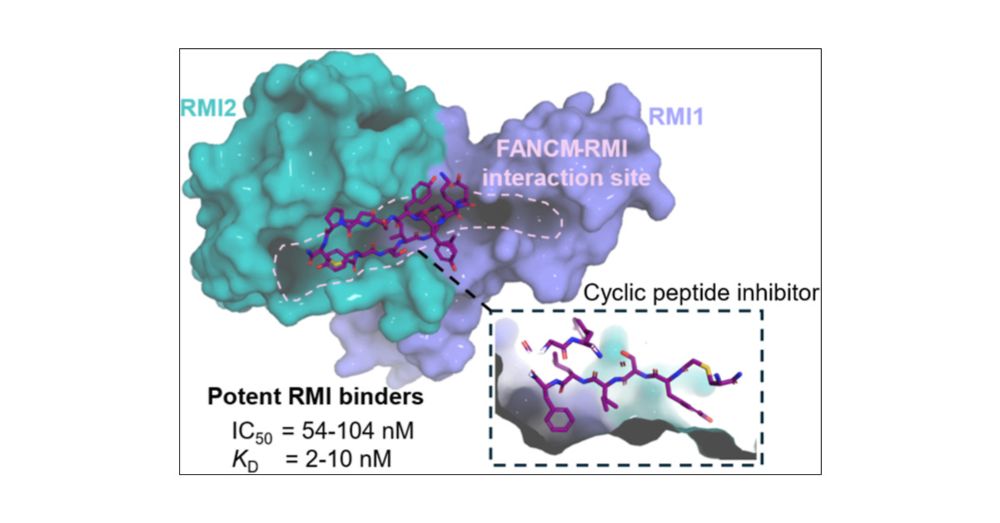
Potent Cyclic Peptide Inhibitors Disrupt the FANCM–RMI Interaction
FANCM–RMI is a protein–protein interaction that maintains genome stability during DNA repair events in cancers that rely on the Alternative Lengthening of Telomeres (ALT) pathway for survival. We repo...
pubs.acs.org
Reposted by Andrew Deans